Keywords: mitral regurgitation; diagnosis; treatment
Abbreviations
LV left ventricle
MR mitral regurgitation
MV mitral valve
TOE transoesophageal echocardiography
TTE transthoracic echocardiography
Take-home messages
How can we recognise the aetiology of primary MR?
Recognising the aetiology of primary MR is important because it may have implications on treatment and prognosis.
The most common aetiology is degenerative [1-2] and can be either a myxomatous degeneration or a fibroelastic deficiency (Figure 1A, Figure 1B; Video 1-2). These diseases can cause various types of lesions known as billowing, prolapse and flail. The term billowing refers to the curvature of the leaflets. It is pathological when the distance between the body of the leaflet and the annulus is more than 2 mm in a long-axis view or more than 5 mm in a 4-chamber view, considering the saddle shape of the mitral annulus. The term prolapse indicates the coaptation point above the mitral annulus. Flail is the eversion of a leaflet, generally associated with chordal rupture. Myxomatous degeneration is characterised by thickening of the leaflets, due to an excess of valve tissue and elongated chordae. When generalised, it is known as Barlow's disease.
Rheumatic mitral disease is characterised by a variable thickening of the leaflets, which occurs especially at the free margin, commissural fusion and a systolic-diastolic movement restriction (Figure 1C; Video 3). Fibrosis of the chordae tendineae is also common. Two types of rheumatic MR can be distinguished. In one form, the fibrosis affects the chordae tendineae afferent to both leaflets, which are hypomobile. In the other form, fibrosis mainly affects the chordae afferent to the posterior leaflet, causing a pseudo-prolapse of the anterior leaflet [3].
Libman–Sacks endocarditis is a form of abacterial endocarditis, also called marantic endocarditis. It is found in association with inflammatory diseases, such as systemic lupus erythematosus, antiphospholipid antibody syndrome and cancer. The vegetations are usually small and are located mainly at the level of the basal and middle portions of the leaflets (Figure 1D; Video 4). Infective endocarditis is recognised by the presence of vegetations, leaflet perforation or perivalvular abscesses (Figure 1E; Video 5) [4].
Congenital forms of MR are cleft (indentation of the leaflet that extends more than 50% of the depth of the leaflet) (Figure 1F; Video 6) and "parachute mitral valve" (when all the chordae originate from a single papillary muscle) [5].
Primary MR is often associated with mitral annulus disease. In Barlow's disease the annulus is generally very dilated and has a reduced protosystolic contraction due to an expansion of the intercommissural diameter [6]. Mitral annular calcification is a chronic degenerative process that mainly affects the posterior part of the mitral annulus. It is often found in elderly and in younger patients with renal failure or arterial hypertension. Caseous mitral annular calcification is a variant of the classic form, characterised by the presence of a colliquative necrosis into the calcific mass. It is generally echoreflective on the periphery and echofree in the centre. The calcific ring has an altered systolic and diastolic movement, reducing the continence of the MV [7].
Mitral annular disjunction is a structural anomaly that consists in a clear separation measured at end-systole between the annulus and the basal inferolateral myocardium of the LV. It may be associated with MV prolapse and curling of the posterior annulus and with an increased risk of ventricular arrhythmias [8]. In patients with MV prolapse, tissue Doppler of the lateral mitral annulus may show an increase in the peak velocity of the S' wave ≥16 cm/s. This increase is called the pickelhaube sign and represents a trigger for generating premature beats [9].
Transthoracic echocardiography (TTE) is generally sufficient to describe valvular anatomy. However, three-dimensional (3D) transoesophageal echocardiography (TOE) can provide an “en face” view of the mitral leaflets resembling the surgical inspection of the valve, thereby facilitating the Heart Team discussion.
Figure 1 and Videos 1-6 . Examples of primary MR.

Video 1
Video 2
Video 3
Video 4
Video 5
Video 6
Panel A and Video 1. Fibroelastic deficiency with prolapse and flail of P2 (3D “en face” view); Panel B and Video 2. Myxomatous degeneration (3D “en face” view); Panel C and Video 3. Rheumatic disease (parasternal long-axis view); Panel D and Video 4. Marantic vegetation on posterior annulus (3D “en face” view); Panel E and Video 5. MR due to endocarditis and leaflet perforation (2-chamber view on TOE, red arrow); Panel F and Video 6. Cleft of the posterior leaflet (3D “en face” view, red arrow).
Which methodology best defines MR severity?
TTE remains the cornerstone to estimate primary MR severity and to evaluate its impact on left atrial, LV and systolic pulmonary artery pressure. An integrated echocardiographic approach based on different parameters is appropriate and an algorithm has been proposed for grading severity into mild, moderate and severe MR (Figure 2) [10].
Figure 2. Multimodality algorithm proposed for grading MR severity into mild, moderate and severe.

CMR: cardiac magnetic resonance imaging; EROA: effective regurgitant orifice area; LV: left ventricle; MR: mitral regurgitation; MV: mitral valve; PAP: pulmonary artery pressure; PV: pulmonary vein; RF: regurgitant fraction; RV: right ventricle; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography; VC: vena contracta
In chronic forms, the severity of MR can be expressed by considering the extent of valve damage, the entity of regurgitation and its haemodynamic consequences. Severe valve lesions, such as flail leaflet, ruptured papillary muscle, severe leaflet retraction, or a large perforation, are specific for severe MR.
There are various qualitative and quantitative methods to evaluate the severity of MR. Quantitative evaluations include calculation of the regurgitant volume and effective regurgitant orifice area using the proximal isovelocity surface area method. A cut-off value of effective regurgitant orifice area ≥0.40 cm2 is consistent with severe degenerative MR. However, there are limitations, which should be taken into consideration. For example, the severity of eccentric regurgitant jets, such as those occurring in localised or commissural prolapse, may be underestimated. Conversely, the severity of regurgitant jets confined at the end-systolic phase of the cardiac cycle may be overestimated.
Regurgitant volume can also be obtained using the echo-Doppler method by calculating the difference between the total and the systemic LV stroke volume. The first is obtained at the MV level as the product of the mitral annulus area and the time-velocity integral of the mitral inflow; the second is calculated by multiplying the LV outflow tract area by the time-velocity integral of the aortic outflow. This method should not be applied in the presence of significant aortic regurgitation. Regurgitant volume values identifying severe MR are ≥60 ml. The regurgitant volume can be expressed as a percentage of the total LV stroke volume, obtaining the regurgitant fraction. The regurgitant fraction is ≥50% in severe MR. When MR is not holosystolic, it is preferable to use the echo-Doppler method which is not affected by the MR duration [10].
Chronic MR can cause volume and pressure overload of the cardiac chambers. Absence of LV and left atrial dilatation should raise doubts as to the severity of MR. In compensated forms, without LV dysfunction, the LV ejection fraction is >60% and the LV end-systolic cavity dimensions (diameter and volume) and stress are generally not increased. In decompensated forms, the LV ejection fraction becomes <60% and the LV end-systolic dimensions and stress increase.
Doppler methods allow indirect assessments of transmitral inflow, left atrial pressure, ventriculoatrial gradient and systolic pulmonary artery pressure. As regards transmitral inflow, the increase in diastolic inflow volume determines the increase in the peak velocity of the E wave (>1.5 m/sec) and an E/A ratio >1 (E dominant pattern). Therefore, an E/A ratio <1 without an increase in the peak velocity of the E wave (A dominant pattern) excludes the presence of severe MR [10].
With severe MR, the characteristics of the pulmonary venous flow pattern are altered, due to the increase in left atrial pressure. In moderate MR, there is a reduction in the systolic wave and an increase in the diastolic wave, with an inversion of the systolic/diastolic ratio. In advanced forms, the absence or inversion of the systolic wave is observed, with a higher diastolic wave. Pulmonary venous flow can be assessed using TTE only at the level of the right superior pulmonary vein, placing the pulsed wave Doppler sample volume within this vessel. This is a limitation when the regurgitant jet reaches the outlet of this pulmonary vein, generating a retrograde systolic signal that can be mistaken for the pulmonary venous one, resulting in a false-positive result. When TOE is performed, this limit is overcome by evaluating the flow velocity in at least two pulmonary veins (one right and one left). A false-negative result may be observed when the compliance of a dilated left atrium is high.
Clinical presentation and the haemodynamic state should be considered when making a final judgment of MR severity, as MR is conditioned by the afterload and heart rate at the moment of the assessment. TOE and cardiac magnetic resonance imaging are indicated to evaluate MR severity in patients for whom TTE is inconclusive or when there is a discrepancy between MR severity and the clinical findings. MR can be assessed by cardiac magnetic resonance imaging using several techniques with quantification of the regurgitant volume and the regurgitant fraction being highly recommended. Exercise echocardiography may be helpful in patients with discordant symptoms and MR grade at rest: the test is reasonable in symptomatic patients with at least moderate MR and in asymptomatic patients with severe MR (Figure 2) [10, 11].
When to operate on patients with severe MR?
Surgery is recommended in symptomatic patients who are operable and not at high risk. In asymptomatic patients with severe MR, surgery is recommended if the LV ejection fraction is ≤60% or an LV end-systolic diameter ≥40 mm. The end-systolic diameter is less powerful as a prognosticator compared to the ejection fraction. Alternative indices could be the end-systolic volume and global longitudinal strain. It has been suggested to consider surgery earlier when the ejection fraction is progressively declining (even above 60%) or if the end-systolic diameter is progressively increasing (even below 40 mm) on serial imaging studies. However, there are no clear indications for the timing of repeated examinations and the imaging technique to utilise for this purpose[12].
TTE is certainly the most used technique in clinical practice but the ejection fraction measurement has a significant error that should be considered when interpreting iterated examinations. Cardiac magnetic resonance imaging and 3D echocardiography are more accurate but, because the error of each technique varies, different techniques are not interchangeable. When there is no clear evidence of LV systolic dysfunction, the choice of surgery for asymptomatic patients should rely on additional factors, in an effort to prevent adverse LV remodelling and dysfunction.
Surgery is favoured when there is an occurrence of new-onset atrial fibrillation secondary to MR and by a systolic pulmonary artery pressure ≥50 mmHg at rest. The left atrial volume should also be taken into account, because a left atrial volume ≥60 ml/m2 or a diameter ≥55 mm identifies patients who do not sufficiently dilate their LV, but dilate their left atrium fully and have a risk of atrial fibrillation.
In patients with severe primary MR, an ejection fraction >60%, no atrial fibrillation and normal resting systolic pulmonary artery pressure, surgery might prevent not only the deterioration of LV function but also the development of atrial fibrillation or pulmonary artery hypertension. In these patients, surgery (MV repair) may be considered only if it is performed at a Heart Valve Centre with an excellent reputation and with a very high likelihood of a successful and durable repair with very low mortality risk [12].
The role of exercise pulmonary hypertension in indicating surgery in primary MR has been debated in recent years and remains controversial. In many asymptomatic patients with severe MR and normal resting systolic pulmonary artery pressure, exercise echocardiography can determine the increase in systolic pulmonary artery pressure. A value of >60 mmHg has been shown to predict the occurrence of symptoms and to identify patients at higher risk. Current guidelines have taken out recommendations for surgery based on exercise pulmonary artery hypertension. Exercise echocardiography may be proposed for asymptomatic patients with severe MR and normal systolic pulmonary artery pressure at rest to identify patients at higher risk. However, a positive result of exercise echocardiography is still not a straightforward indication for surgery in asymptomatic patients with normal LV function and resting systolic pulmonary artery pressure and should be discussed by the Heart Team, particularly for patients with an abrupt increase in systolic pulmonary artery pressure at low levels of exercise and with posterior MV prolapse [13]. If none of the above indications for surgery are present, a watchful waiting strategy can be safe. Figure 3 summarises when to operate patients with MR.
Figure 3. Summary of indications for MR treatment.
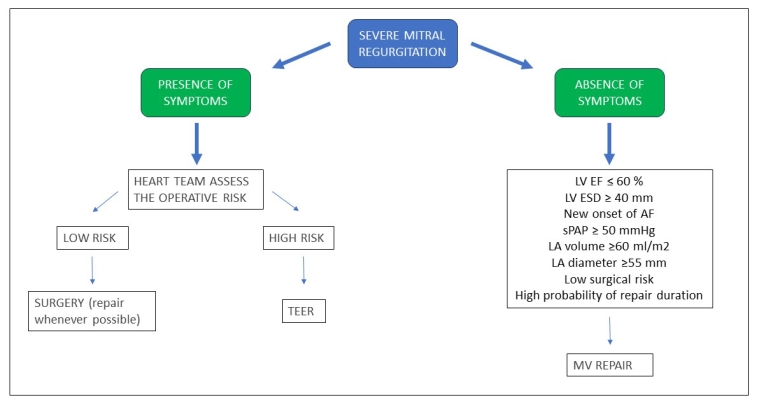
AF: atrial fibrillation; LA: left atrium; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; MV: mitral valve; sPAP: systolic pulmonary artery pressure; TEER: transcatheter edge-to-edge repair
How to perform surgical repair and predict results?
MV repair is the preferred surgical approach because it is associated with lower operative mortality, better long-term survival, and fewer valve-related complications compared with MV replacement. Successful repair can be predicted using cardiac imaging criteria (Table 1). Predictive factors of suboptimal repair of degenerative MR are the presence of a large central regurgitant jet, the involvement of 3 or more scallops (especially if the anterior leaflet is involved), extensive dilatation and calcification of the mitral annulus. In valvular endocarditis with destructive lesions of the leaflets, the lack of tissue is a further predictor of suboptimal repair. Repair of rheumatic MV disease should be limited to patients with a very high probability of successful and durable repair especially in the younger age group or when replacement cannot be performed (usually because of anticoagulation problems) [14].
Table 1. Cardiac imaging criteria for predicting successful repair of MV.
| Aetiology | Morphology | Calcification | Annular dilatation | Repair |
| Degenerative | P2 and/or prolapse | No/limited | Mild/moderate | Feasible |
| ≥3 scallops or anterior commissure prolapse | Limited (annulus) | Moderate | Difficult | |
| ≥3 scallops or anterior commissure prolapse | Extended (annulus+leaflets) | Severe | Unlikely | |
| Rheumatic | Mobile anterior leaflet | Limited | Moderate | Difficult |
| Rigid anterior leaflet | Extended (annulus+leaflets) | Moderate/severe | Unlikely | |
| Endocarditis | Destructive lesions | No | No/mild | Unlikely |
A variety of repair techniques exist and different paradigms have emerged over the years. One is based on Carpentier’s technique, which generally involves resection of abnormal tissue with reconstruction toward a normal valve function. A different approach (“respect rather than resect” tissue) uses artificial neochordae to reconstruct support of the free edge of prolapsing segments, “displacing” abnormal excess tissue into the LV to ensure a good surface of coaptation. Another approach is the edge-to-edge MV repair by Alfieri, especially helpful in case of commissural lesions and in patients suffering from Barlow disease.
Regardless of the leaflet and chordal techniques applied, an annular prosthesis (complete ring, partial posterior band; rigid, semi-rigid, or flexible) is a mainstay of all repair procedures to restore the normal circumference and shape of the MV. The size of the ring is measured according to the size of the anterior leaflet and should not be undersized. If repair is not feasible, MV replacement with preservation of the subvalvular apparatus should be pursued and a bioprosthesis should be favoured in elderly patients.
Minimally invasive approach to MV surgery based on minithoracotomy is performed more frequently to decrease surgical trauma and accelerate postoperative recovery. The right anterolateral minithoracotomy in the fourth intercostal space is currently the most common approach. Endoscopic use or robotic use allow truly minimal invasive repairs but are not always used in conjunction with minithoracotomy. Results of the minimally invasive approach may be similar to those of the conventional sternotomy in the hands of highly experienced surgeons and in selected patients [15].
Transapical off-pump NeoChord (NeoChord) repair is a novel TOE-guided minimally invasive surgical procedure to treat degenerative MR through a left lateral minithoracotomy in the fifth intercostal space. After ventriculotomy, the device is inserted in the LV and guided to the prolapse/flail leaflet and a number of neochordae are implanted and properly tensioned to restore adequate coaptation and are then secured to the LV epicardium. This procedure is notable for the lack of an annular prosthesis. The NeoChord repair system is an option for patients with isolated central or multisegment posterior leaflet prolapse/flail and anterior leaflet disease if adequate MV tissue overriding is present, although it is not recommended in cases of paracommissural disease, and/or calcifications of the annulus/leaflets, after excluding functional components. Echocardiography can help to predict the success of the NeoChord repair by using quantitative indices [16].
Finally, echocardiography is useful for preoperatively identifying those patients at higher risk of developing a systolic anterior motion of the mitral anterior leaflet, and thus LV outflow tract obstruction, after MV repair or replacement [17].
When to perform percutaneous repair and how to predict results?
Several transcatheter MV interventions have been proposed for the treatment of MR. At present, edge-to-edge MV repair is used most frequently. This procedure approximates the free edges of the anterior and posterior MV leaflets at the site of the MR jet and emulates surgical edge-to-edge leaflet repair (also called the “Alfieri stitch”), which is differentiated by the concomitant annuloplasty.
A number of echocardiographic evaluations are performed to identify the best candidates, including prolapse/flail location, flail gap, flail width, posterior leaflet length and MV orifice size [10]. After the initial experiences in treating simple A2-P2 related MR, the repair of more complex lesions is now performed more frequently.
Today, percutaneous options can only be proposed to patients with symptomatic primary severe MR at high risk for conventional surgery or who are deemed inoperable [12]. Experience with other catheter-based approaches for MR treatment (transcatheter MV replacement, annuloplasty devices and/or insertion of artificial chordae) is still limited and no suggestions or recommendations can be currently provided.
How to follow up operated and non-operated patients?
Asymptomatic patients with severe primary MR, an LV ejection fraction >60% and an end-systolic diameter <40 mm should be followed-up with a cardiological examination and TTE every 6 months. If, during follow-up, the end-systolic diameter increases or the ejection fraction reduces, the surgical indication for valve repair must be rediscussed. Asymptomatic patients with moderate MR and an ejection fraction within normal limits can be followed-up with clinical re-evaluations every year and TTE re-evaluations every 1-2 years.
After intervention, serial follow-up focuses on evaluation of symptomatic status, presence of arrhythmic events, assessment of valve function and recurrence of MR. After transcatheter MV repair, yearly TTE is appropriate [12].
Impact on practice statement
Today the approach to primary MR is multimodal and multiparametric. It must be integrated with the evaluation of the overall cardiac function and the patient's clinical status in order to define the indications, timing and type of intervention.




 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.