Objectives of the meeting
Historical Development of Pharmacotherapy
Pharmacotherapy started with herbs that were sometimes useful, but often toxic. Then we moved to pills, where the active ingredient (for example for digitalis, the steroid) was purified and a more precise dosage and avoidance of side-effects, as much as possible, became a reality. Pharmacotherapy today relies on pills containing a vast number of molecules interfering with cell surface receptors (e.g. a/b-receptors, AT1-receptors, P2Y12-receptors), enzymes (ACE, neprilysin), ion/metabolite transporters (SGLT2) and other pathways for the treatment of blood pressure, lipids, diabetes, coronary artery disease and heart failure. More recently, antibodies interfering with crucial proteins have become clinical practice, after rheumatology (infliximab, adalimumab, among others) now also in cardiology with antibodies against PCSK9.
Nucleotid acids as novel therapeutics
A true revolution of pharmacotherapy took place with the discovery of RNA interference by Andrew Fire and Craig Mello, Nobel Prize Laureates in 2006, as well as the use of antisense oligonucleotides. These molecules no longer interfere with surface receptors or enzymes in the circulation or in cardiovascular tissue, but rather prevent the translation of messenger RNA into a target protein (Figure 1). When combined with tags interfering with specific surface receptors (e.g. GalNac with Asialoglycoprotein receptors on hepatocytes) these nucleic acids can be made highly organ and tissue specific and often have a long duration of action (up to >6 months).
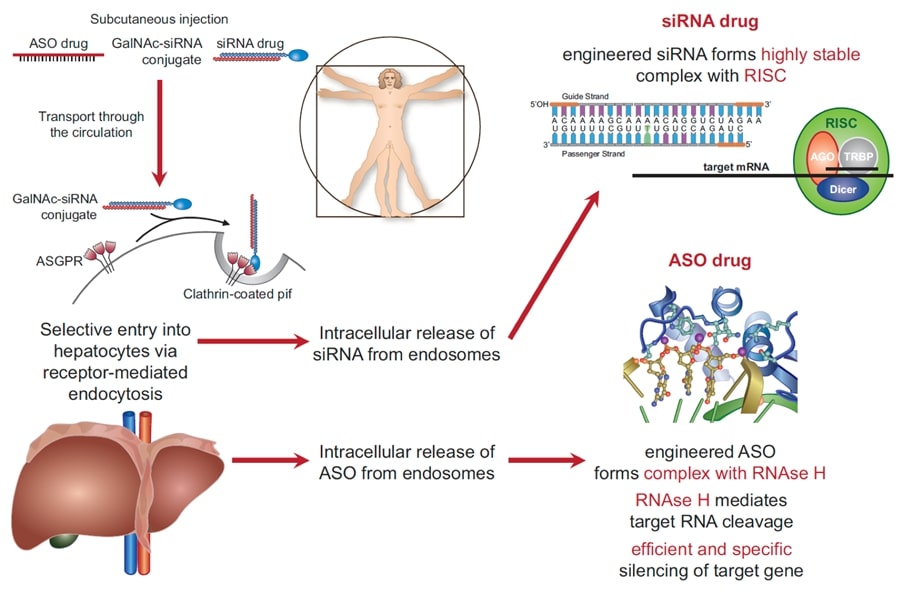
Figure 1: Mechanisms of action of RNA interference in the liver. The double strand RNA with a nucleotid sequence complementory to the target mRNA is tagged with N-acetylgalactosamine (GalNac) and binds speficially to the Asialoglycoprotein receptor (ASGR) that is only expressed in liver cells. After internalisation, it binds in the cytoplasm to the RNA-Induced Silencing Complex (RISC) and prevents translation of the transcript to the target protein for prolonged periods of time. (from Landmesser U, …Luzscher TF. Eur. Heart J. 2020;44:3848-3899).
CRISPR-Cas9: From treatment to cure
Of note, the future holds even more promising tools; indeed, with the discovery of the gene scissor, CRISPR-Cas9, by Emmanuelle Charpentier and Jennifer Doudna, Nobel Laureates in 2020 (Figure 2) gene editing has become possible with a single treatment in a lifetime for pa-tients with various medical conditions. Indeed, in Amyloidosis already a few patients have been successfully treated and as for lipids, the technique has been successfully used in primates.

Figure 2: The discovery of geneediting with CRISPR-Cas9
These new developments in pharmacotherapy bring enormous opportunities, but also questions as regards their safety and sustainability, but they will certainly change medicine as it has been practiced in the past.
Objectives of the CRT meeting: To discuss the basics of this new pharmacotherapy, its current application and future perspectives, as well as important safety and ethical issues associated with it. Furthermore, the goal is to involve the European Medical Agency (and possibly the Federal Drug Administration in the USA) to discuss registration issues with these new technologies, and in particular, as regards the advent of CRISPR-Cas9.
Related Reading
Session Recording
Day 1
Day 2
Final Programme
Academic Chairpersons
- Prof. Ulf Landmesser
- Prof. Thomas F. Lüscher (ESC President-elect and ESC Chair of the CRT)
Industry Chairpersons
- Dr. Paul Nioi (Alnynam)
- Dr. Andres Laguna (Novartis)
- Dr. Jürgen Prochaska (Boehringer Ingelheim Pharmaceuticals Inc)
- Dr. Ricardo Rocha (Intellia Therapeutics)
- Prof. Alexandra Goncalves (BMS) (Industry Chair of the CRT)
DAY 1: 31 January 2024 - 14:00-18:15
| Time | Session | Speaker |
|---|---|---|
|
12:30-14:00 |
Arrival and buffet lunch |
|
|
14:00-14:05 |
Welcome from the CRT Chairpersons |
Thomas F. Lüscher Alexandra Goncalves (BMS) |
|
14:05-14:15 |
General introduction to the workshop |
Ulf Landmesser Thomas F. Lüscher
|
|
14:15 -18:30 - SESSION 1 – NucleIC Acids: Concept and Potential Moderated by : Francesco COSENTINO and Alexandra Goncalves (BMS) |
||
|
14:15-14:30 |
From Herbs to pills, antibodies and nucleic acids: The rise of pharmacotherapy |
|
|
14:30-14:50
|
Efficacy and safety of siRNA and ASO therapeutics |
|
|
14:50-15:10 |
The future of nucleic acid therapeutics: The industries view |
|
|
15:10-15:35 |
Nucleic acid-based therapeutics for CVDs: safety insights from EMA Scientific Advice and marketing authorizations |
Gabriella Passacquale, Anna Tavridou, and Clemens Mittmann (EMA) via Zoom |
|
15:35 - 15:50 |
Coffee break |
|
|
15:50-16:50 - BREAKOUT SESSIONS 1: The future of nucleoid acids - 60 min |
||
|
|
Group 1: Ethics
|
Lead: Julian März Rapporteur: Jürgen Prochaska (BI) |
|
|
Group 2: Safety and Regulatory approval
|
|
|
|
Group 3: Future perspectives from the medical side |
Lead: Francesco Cosentino |
|
17:00 - 17:10 |
Report from breakout session 1 – 10mins |
Rapporteur: Jürgen Prochaska (BI) |
|
17:10 - 17:20 |
Report from breakout session 2 – 10mins |
Rapporteur: Paul Nioi (Alnynam) |
|
17:20 - 17:30 |
Report from breakout session 3 – 10mins |
Rapporteur: Anders Himmelmann (AstraZeneca) |
|
17:30-18:00 |
How do we implement new therapeutics to change the paradigm? |
|
|
18:00-18:20 |
Wrap-up and summary Day 1 – Outlook to Day 2 |
Chairpersons |
| END OF DAY 1 | ||
|
20:00 |
Dinner |
|
DAY 2: 01 February 2024 - 08:00 - 12:45
| Time | Session | Speaker |
|---|---|---|
| 08:00 - 12:45 - SESSION 2 – From Antibodies to Nucleotid Acids and beyondModerated by : Ulf Landmesser and Jürgen Prochaska (Boehringer Ingelheim Pharmaceuticals Inc) | ||
| 08:00-08:30 | Cardiovascular Gene Therapy - Efficient gene delivery to the heart? What are the best targets? | Roger Hajjar |
| 08:30-09:00 | ASO/siRNa vs. antibodies for TTR amyloid heart disease | Mathew Maurer |
| 09:00-09:30 | miRNAs as therapeutic tools | Thomas Thum |
| 09:30-10:00 | CRISPR-Cas9: From treatment to cure? | Sekar Kathiresan (Verve Therapeutics) |
| 10:00 - 10:15 | Coffee break | |
| 10:15 - 11:15 - BREAKOUT SESSIONS 2: From Treatment to cure - 60 min | ||
| Group 1: Compliance with the new tools | Lead: Francesco Cosentino Rapporteur: Ricardo Rocha (Intellia Therapeutics) |
|
| Group 2: miRNAs | Lead: Christopher O’Donnell (Novartis) Rapporteur: Tomasz Guzik |
|
| Group 3: How to target the heart or the kidney: in search of novel strategies | Lead: Benjamin Meder Rapporteur: Lothar Roessig (ASKBIO/Bayer) |
|
| 11:15-11:25 | Report from breakout session 1 – 10mins | Rapporteur: Ricardo Rocha (Intellia Therapeutics) |
| 11:25-11:35 | Report from breakout session 2 – 10mins | Rapporteur: Tomasz Guzik |
| 11:35-11:45 | Report from breakout session 3 – 10mins | Rapporteur: Lothar Roessig (ASKBIO/Bayer) |
| 11 :45-12:05 | Patient’s perspectives on nucleoid acid therapy | Mattias Van Heetvelde (ESC Patient Forum) |
| 12:05 – 12:35 | Ethical issues of genetic therapeutic tools | Julian März |
| 12:35 – 12:45 | Wrap-up, conclusions, next steps for a publication | Chairpersons |
| END OF DAY 2 | ||
| 13:00 | Buffet lunch and departures | |
Biographies
- Prof. Thomas F. LÜSCHER
- Prof. Alexandra GONCALVES
- Prof. Ulf LANDMESSER
- Dr. Andres LAGUNA
- Dr. Jürgen PROCHASKA
- Dr. Mathew S. MAURER
- Prof. Anders HIMMELMANN
- Dr. Christopher O'DONNELL
- Dr. Sekar KATHIRESAN
- Dr. Julian MÄRZ
- Prof. Thomas THUM
- Prof. Benjamin MEDER
- Prof. Roger HAJJAR
- Dr. Lothar ROESSIG
- Prof. Lale TOKGÖZOĞLU
- Dr. Anna TAVRIDOU
- Dr. Gabriella PASSACQUALE
- Mr. Mattias VAN HEETVELDE
- Prof. Francesco COSENTINO
- Dr. Clemens MITTMANN

 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.