Take-home messages
- Heat exposure is associated with increased risks for cardiovascular mortality and morbidity.
- Age, pregnancy, pre-existing health conditions, occupational heat exposure, low socioeconomic status, and unfavourable living environments confer greater vulnerability to heat.
- Heat-related cardiovascular impact is predicted to increase in the future due to the combined effects of global warming, population growth and ageing.
- Potential pathophysiological mechanisms underlying heat impact includes increased cardiac load, reduced blood pressure, prothrombotic conditions, and systemic inflammatory responses.
- Heat-health action plans need to be implemented at national and institutional levels (hospitals, care homes, and emergency services).
Introduction
High air temperatures on single hot days and during heatwaves pose risks to health, either directly by causing dehydration or heatstroke, or indirectly via pathways such as worsening of cardiorespiratory diseases (CPD), kidney diseases, or electrolyte disorders. Individuals suffering from pre-existing diseases, especially CPD, are at high risk of heat-related health problems, leading to increased emergency room visits and hospital admissions. Age, socioeconomic status, as well as underlying health conditions increase the risk of heat-related acute cardiovascular disease (CVD) events such as myocardial infarction (MI) [1]. Additionally, adverse environmental conditions, especially air pollution, have an interactive effect on heat-related health effects [2]. Megatrends, such as population growth and ageing, urbanisation, and socioeconomic development pathways further promote vulnerability to heat [3]. Overall, CVD events are the primary cause of death during heatwaves.
This review highlights the current epidemiological evidence for the adverse effect of heat on CVD events and factors characterising heat vulnerability, and discusses future projections of temperature-related health impact. We also address the potential pathophysiological mechanisms underlying the cardiovascular effects of heat, as well as the interaction between heat and cardiovascular drugs. Moreover, we provide suggestions regarding how cardiologists can be engaged in heat-health action plans and deliver effective care to their patients during heat exposure.
Heat effects on cardiovascular mortality and morbidity
Short-term associations between high temperatures (heat) and cardiovascular mortality have been established in numerous studies. A meta-analysis by Bunker et al in 2016 [3] found that a 1°C rise in temperature increased cardiovascular mortality by 4.15% (95% confidence interval [CI] 3.70–4.59) and cerebrovascular mortality by 2.59% (95% CI: 1.46–3.73) within two days after exposure in the elderly (>65 years). In contrast, associations between high temperatures and cardiovascular morbidity were less conclusive. For example, a meta-analysis by Sun et al in 2018 [4] reported a 1.6% (95% CI: 0.4–2.8) increase in MI hospitalisation associated with a 1 °C rise in temperature, whereas no significant heat effects on cardiovascular admissions were found in the meta-analysis by Bunker et al [3].
Previous studies have shown geographical variations in heat-related risks. In a multiple country study, Guo et al (2017) [5] observed stronger heatwave effects on mortality in moderately cold and moderately hot areas than in cold and hot areas. Another study conducted in the US found greater mortality responses to heatwaves in the Northeast and Midwest than in the South [6]. These findings indicate different temperature adaptations of the populations.
In addition, there is evidence suggesting temporal variations in cardiovascular effects of heat over the past decades. For example, using the MI registry data from Augsburg, Germany, Chen et al (2019) [7] reported higher heat-related MI risk in 2001-2014 compared to 1987-2000. The increasing heat-related MI risk over time can be explained by the relatively temperate but warming climate in Augsburg, where residential air conditioning is not widely used, as well as increasing vulnerability due to a rising prevalence of MI risk factors like diabetes and hyperlipidaemia.
The effects of heat on health mainly occur within a week after heat events. Some studies documented mortality displacement, characterised by excess deaths that occur shortly after heat exposure, followed by a period of lower-than-average mortality. This observation is consistent with a temporal advance in deaths among highly vulnerable individuals that would have happened later in the absence of initial heat stress. To assess the impact of short-term mortality displacement, several studies examined the long-term health effects of heat using indices of annual heat exposure. They found increases in mortality associated with heat as well, suggesting most deaths attributable to heat in daily analyses were advanced by at least one year [8].
In addition to independent heat effects, joint heat and air pollution effects on cardiovascular mortality were observed. A study involving eight European urban areas shows that high concentrations of particulate matter and ozone enhanced heat effects on cardiovascular mortality [2]. For example, heat exposure was associated with an increase in the cardiovascular mortality risk by 7.04% (95% CI: 0.51–9.69) at low PM10 (particulate matter with aerodynamic diameter ≤ 10 µm) levels and by 13.69% (95% CI: 1.84–26.91) at high PM10 levels.
Vulnerability to adverse heat effects
It has been recognised that the distribution of adverse heat effects varies across populations. Specific subgroups are at a greater risk of heat-related mortality and morbidity due to individual or contextual characteristics (Figure 1). A systematic review and meta-analysis of 61 studies on vulnerability to heat-related mortality found the strongest evidence for older age (age >65 years) [9]. The vulnerability of older adults is partly attributable to comorbidities, use of medications, and a decline in thermoregulatory function, such as reduced skin blood flow and sweat rate [3].
People with underlying medical conditions, including CVD, lung diseases, diabetes, neurodegenerative diseases such as dementia and Parkinson’s disease, and mental health issues, have demonstrated greater vulnerability to heat stress as well. Moreover, pregnant women are at higher risk during heat exposure due to increased heat production, which results from body weight gain and metabolic demands of the growing foetus. Furthermore, they are more susceptible to dehydration.
In addition to the direct health effects on pregnant women, heat has been identified as a risk factor for adverse birth outcomes, including preterm birth, low birth weight, stillbirths, and congenital heart defects [10]. In the occupational setting, prolonged heat exposure and intensive physical work can lead to more heat stress for workers, even at temperatures lower than those that trigger heatwave alerts. Therefore, occupational heat strain needs to be recognised when assessing the heat-related risk of workers.
Figure 1. Individual and contextual factors influencing vulnerability to heat.
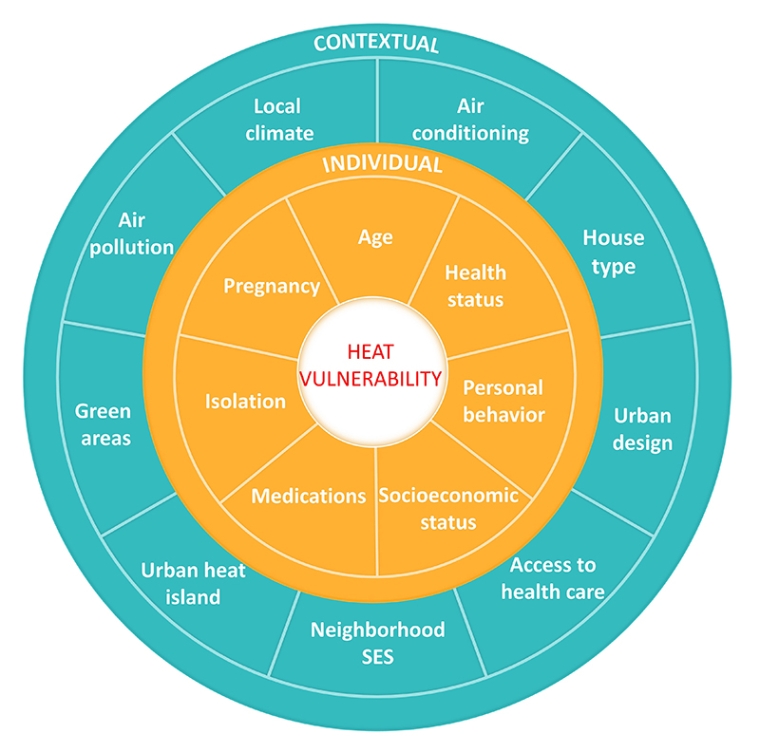
SES:socioeconomic status
Contextual characteristics, such as lower socioeconomic status and unfavourable living environments, are also among the factors that confer a greater vulnerability to heat. The vulnerability of disadvantaged people might be explained by their reduced capacity for adaptation, for instance, poor health status, lifestyle risk factors, lack of access to air conditioning, and living in densely populated cities.
The effect of urban heat islands is of concern considering the increase in urbanisation worldwide. The urban heat island effect is characterised by higher air temperatures and smaller diurnal temperature variations in urban areas relative to outlying areas, due to limited natural landscapes and urban building materials that retain heat and alter the microclimate. This might contribute to stronger adverse heat effects among urban populations. In urban areas particularly, night-time heat exposure is exacerbated by the urban heat island effect [11]. In the future, night-time warming is projected to surpass daytime warming in many regions across the world [12]. Among the factors affecting sleep, night-time temperature plays an important role. The findings of a recent study suggest a connection between hot nights and mortality in areas of Southern Europe [11]. Furthermore, the heat effects in urban areas could be exacerbated by larger socioeconomic disparities and concurrent exposure to air pollution.
It is essential to identify and localise vulnerable subgroups in order to help target interventions that protect those most at risk during hot days and heatwaves. Accordingly, several studies have developed heat vulnerability indicators that combine area-level sociodemographic, health status, and environmental factors. By visualising the vulnerability scores via mapping, areas where people are vulnerable to heat can be identified. In the 2021 report of the Lancet Countdown on health and climate change, a global heat vulnerability index was developed, combining the country-level proportion of the population aged over 65 years, the prevalence of cardiorespiratory disease and diabetes, and the proportion of the population that lived in urban areas. Other heat vulnerability index maps with a finer resolution, such as at the census tract or block group level, have been developed in North American, European, and Oceanian cities.
Future projection of temperature-related cardiovascular impact
Projection studies on temperature-related cardiovascular impact has been growing in recent years [13-16]. Despite projection studies on CVD outcomes being rather limited to selected cities or regions, most studies provide evidence of increased temperature-related CVD outcomes in the future as a result of climate change.
A study in Beijing projects increases in temperature-related CVD mortality by up to 10.2% under different climate scenarios until 2070 [13]. Similarly, a study in Augsburg, Germany, concluded that temperature-related MI cases would increase by 18% and 63% per decade with a warming of 2°C and 3°C, respectively [14]. On the other hand, there are arguments that cold-related CVD outcomes would decrease in the future because of climate change, which might outweigh the increase in heat-related outcomes [17]. However, a study conducted in Brazilian cities expects the increase in CVD mortality related to high temperatures to outweigh the reduction in mortality related to low temperatures, mainly only under the high emission scenario - representative concentration pathway (RCP) 8.5 [16].
Nevertheless, none of the aforementioned studies considered the future population change or population ageing, both of which are major factors in determining future temperature-related health outcomes. This, in turn, might have led to an underestimation of future temperature-related impact. A study by Zhang et al (2018) [13] demonstrated that when population change was taken into consideration, the annual rate of increase in temperature-related CVD deaths was up to fivefold greater than the rate under the no-population-change scenario. Similarly, another study showed that when considering demographic change, the temperature-related disease burden of ischaemic heart diseases in the elderly would be exacerbated by up to 196.6% under different RCPs in the 2070s; the increase is much higher than the calculated 38.3% under a similar scenario that did not consider future demographic changes [15].
Furthermore, future temperature-related CVD outcomes are largely dominated by future shared socioeconomic pathway (SSP) scenarios [18]. A comprehensive study examining mortality by individual cause category, world region and SSP through 2080, illustrates that CVD will continue to be the single leading cause category of death worldwide, constituting over 50% of all deaths in Europe and Central Asia and about 42% in the Middle East and North Africa under all SSPs by 2030 [18]. Elevated CVD mortality in these regions is consistent with projections suggesting rising obesity levels, particularly under SSPs that assume faster development trajectories [18].
Pathophysiological mechanisms
Studies have proposed several pathophysiological mechanisms that might explain the associations between heat exposure and cardiovascular events [1]. Heat exposure can increase skin blood flow and sweating to lower the body’s core temperature. In response, the sympathetic nervous system is activated to maintain cardiac output through a compensatory increase in cardiac workloads, such as increases in heart rate and stroke volume. Peripheral vasodilatation and dehydration might cause drops in blood pressure as well. Furthermore, heat stress is associated with haemoconcentration and a prothrombotic condition due to decreased plasma volume as a result of sweating, and increases in circulating levels of platelets, red blood cells, and blood viscosity.
In addition, heat stress has been shown to induce the release of cytokines such as interleukin 1 and interleukin 6, which modulate local and systemic inflammatory responses and can lead to endothelial dysfunction. The heat-related inflammatory response might be further exaggerated by a bacterial infection following gastrointestinal ischaemia and intestinal hyperpermeability, which is due to decreased splanchnic blood flow to maintain blood pressure during heat exposure [19].
Heart failure (HF) has been identified as an independent prognostic factor in heatstroke-related deaths. Patients with HF are prone to lack the ability to compensate for the increased cardiovascular demands induced by heat exposure. Increased cardiac strain and output, arrhythmias, and peripheral oedema may render HF patients vulnerable to heat-related sudden death and in-hospital mortality. In HF patients, higher temperatures were associated with increased levels of B-type natriuretic peptide and C-reactive protein, both of which are predictors of HF prognosis and severity [20].
The systemic impact of elevated air temperature was also demonstrated by studying plasma metabolites that are associated with cardiovascular diseases. In a study of 2,869 patients who underwent cardiac catheterisation, Hampel et al (2006) [21] found that higher air temperature was associated with increased concentrations of glycine and acylcarnitine (here C16-OH:C14:1-DC). Glycine is a proteinogenic amino acid. Compared to healthy individuals, patients with heart failure or arterial fibrillation are likely to have increased glycine levels. Acylcarnitines are involved in the fatty acid catabolic process in the mitochondria. Higher acylcarnitine concentrations have been associated with mitochondrial dysfunction and an increased risk for mortality.
Heat-drug interaction
Heat has been shown to have potential interactions with cardiovascular drugs. On one hand, heat can affect drug absorption, distribution, and elimination, and subsequently, the therapeutic response. In addition, heat could influence the human body’s adaptation mechanisms, leading to increased risk for drug side effects. For example, heat-related vasodilation can significantly enhance the blood pressure-lowering effects of cardiovascular drugs. This might result in syncope with potentially serious injuries or even myocardial ischaemia [22].
On the other hand, some drugs can interfere with normal thermoregulatory function in various ways, including changes in cardiac output and sweat rate, peripheral vasodilation, and dehydration. It is of note that anticholinergic drugs inhibit thermoregulation, which can reduce sweat rate and cause hyperthermia at extreme temperatures. Diuretics, commonly prescribed for patients with hypertension or heart failure, could exaggerate the adverse effects of heat by inducing dehydration and electrolyte imbalance.
Growing evidence from epidemiological studies suggests the interactive effect of heat and drugs on hospitalisations and mortality. In a matched case-control study, of 1,405 patients admitted to an emergency department in France during the August 2003 heatwave, 4% were diagnosed with hyperthermia or heatstroke (cases) [23]. Compared to the community controls who were not hospitalised, the cases with heat-related pathologies had a higher prevalence of using anticholinergics, suggesting that anticholinergic drugs were an independent risk factor for hospitalisation of heat-related diseases during the heatwave. Another study of 345 patients admitted with heatstroke during the 2003 heatwave in France showed that the use of diuretics was associated with a higher risk for hospital mortality [24].
Consultation room advice
Patients may be encouraged to increase their fluid intake during heat waves. The medication groups that can most often cause problems with heat waves are: diuretics, especially loop diuretics which can cause electrolyte disturbance and reduced fluid volume. ACE inhibitors can also cause dehydration and renal failure. Anticholinergics may cause dry mucous membranes and reduced sweating. Neuroleptics may disrupt the body's temperature regulation and reduce sweat production. Antidepressants can increase the risk of complications through their anticholinergic effect and SSRIs in combination with diuretics increase the risk of hyponatremia. Antihypertensives (including beta-blockers) may contribute to insufficient cardiac output. Antihypertensive and anti-angina preparations reduce arterial pressure, which can cause inadequate heat regulation via impaired sweat gland function. Lithium, digoxin, anti-epileptics and Parkinson's drugs all have a narrow therapeutic range and dehydration can cause serious side effects. NSAIDs may lead to severe kidney failure in the elderly with renal impairment and dehydration. Thus, the dose of certain medicines may need to temporarily be adjusted during periods of high temperatures.
Heat-health action plans
The implementation of heat-health action plans is recommended to prepare for and to prevent the health effects of heat, including access to cool places, behavioural advice, adjustments in medication, and effective treatment for patients at cardiovascular risk [25]. These plans are intended to be enacted from the national to the institutional level (hospitals, care homes, and emergency services) and contain concrete measures to be implemented before the summer, during the summer, and in case of a heatwave, linked to a meteorological early warning system [25]. Capacity needs to be built among health professionals and rescuers on heat-related health outcomes to ensure their diagnosis, consideration of underlying diseases, and timely and effective treatment.
Patients at risk are likely to take medication to treat underlying CVD and related risk factors. Heat-related risks of interactions, loss of effectiveness, adverse side effects, and a reduction in sweating are known among commonly prescribed drugs such as general anticholinergics, antihypertensive or antiarrhythmic drugs, antianginal agents, diuretics, antidepressants, but also insulin or certain analgesics [22]. Thus, medical practitioners need to identify at-risk patients, provide behavioural advice and potentially adjust their medication in view of high temperatures and heatwaves. Recommendations need to include advisable storage temperatures for medications (<25oC max.). Cardiologists are encouraged to offer specific consultations concerning the health effects of climate change and a specific pre-summer season medical assessment with advice relevant to heat (such as hydration advice and medication adjustments) to their patients to raise their awareness and protection capacity
Prevailing research questions need to be urgently addressed, integrated early warning and disease surveillance strengthened, heat-related health protection measures implemented systematically, and their effectiveness assessed in order to prevent the increase of heat-related health hazards and associated morbidity and mortality. Research needs to include monitoring of health status via wearable devices and ambulatory measurements of blood pressure, arrhythmia or dysglycaemia and potential interactions with specific medications and accompanying comorbidities, especially type 2 diabetes and respiratory diseases [1]. Systematic studies on the exact effects of temperature on medications are urgently needed, as no empirical evidence exists for most drugs.
Conclusions
Recent evidence indicates that heat exposure contributes significantly to the exacerbation of cardiovascular disease. The heat-related burden of disease is expected to increase because of global warming, population growth and ageing, and urbanisation. This highlights the urgent need to implement heat-health action plans at national and institutional levels in order to reduce the adverse effects of heat on health.












 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.