Keywords: aetiology, epidemiology, grading, tricuspid regurgitation
Abbreviations
RV: right ventricle
TR: tricuspid regurgitation
Epidemiology of tricuspid regurgitation
Tricuspid regurgitation (TR) is often an incidental finding on routine echocardiography. While a mild degree is generally regarded as a benign physiological entity, moderate and severe TR are associated with worse outcome. The Framingham Heart Study, a large cardiovascular cohort study, showed that the presence of any severity of TR (ranging from trace to more than moderate) was 82% in men and 85.7% in women [1]. The progression from a mild to significant degree of TR is influenced by age and gender. With increasing age, the prevalence of significant TR increases, and in men and women aged >70 years the prevalence of moderate and severe TR reaches up to 1.5% and 5.6%, respectively [1]. Other echocardiographic database studies have reported even higher frequencies of significant TR (up to 16%), which may indicate the varying methodology in grading TR severity in the current literature. In the USA, the prevalence of moderate and severe TR is estimated to be around 1,600,000, far exceeding the estimated number of patients being surgically treated today (8,000/year) [2].
Aetiology of tricuspid regurgitation
In general, the aetiology of TR is classified according to the presence of primary tricuspid valve pathology (primary TR), the presence of associated left-sided heart disease (secondary TR) or the presence of atrial fibrillation without any associated left-sided heart disease (isolated TR). Whereas reported estimated frequencies of primary TR are 8-10%, secondary TR constitutes the predominant mechanism in >90% and, more frequently, isolated TR is now being recognised as a distinct entity (Figure 1).
Figure 1. Causes of tricuspid regurgitation: organic, functional and isolated tricuspid regurgitation.

ARVC: arrhythmogenic right ventricular cardiomyopathy; ICD: implantable cardioverter defibrillator; LV: left ventricle; RA: right atrium; RV: right ventricle; TR: tricuspid regurgitation; TV: tricuspid valve
Primary/organic TR
Primary TR is caused by an abnormality of the tricuspid valve and/or its subvalvular apparatus (tricuspid leaflets, chordae, papillary muscles, or annulus), due to congenital or acquired causes. Acquired causes of primary TR include tumours (carcinoid disease, myxoma), drug-induced leaflet damage (ergot alkaloids, dopamine agonists, anorectic drugs), iatrogenic injury (transvenous pacing or defibrillator leads, endomyocardial biopsy), endocarditis, systemic diseases (lupus erythematosus, sarcoidosis), radiation, rheumatic disease, and trauma [3]. One of the most frequent aetiologies of acquired primary TR is the presence of transvenous leads traversing the tricuspid valve. The pacemaker lead may directly affect the motion of one or more leaflets, interfere with the subvalvular apparatus, or perforate the leaflets, precluding adequate leaflet coaptation. With increasing global numbers of patients with pacemaker and defibrillator devices, the prevalence of lead-induced TR may increase considerably in the near future. In retrospective studies, up to 38% may evolve from trace TR to significant TR over the 1-1.5 years following lead insertion [4]. A multicentre prospective study is currently ongoing, providing systematic one-month and one-year echocardiographic follow-up after device implantation [5]. One of the most frequent congenital causes of TR is Ebstein’s anomaly, which is typically characterised by displacement of the leaflets towards the apex, arising directly from the wall of the right ventricle (RV) without identifiable chordae [6].
Secondary TR
In secondary TR, the underlying mechanism is characterised by RV dilation and dysfunction, leading to leaflet tethering, tricuspid annulus dilation and leaflet malcoaptation [7]. This is most often caused by significant left-sided valvular and myocardial disease, which leads to increased left-sided pressures, pulmonary hypertension, increased RV afterload and remodelling of the RV (Figure 2, panels A and B). In patients with severe mitral regurgitation or severe aortic stenosis, moderate and severe TR is present in more than 25% and 40%, respectively [8,9]. In case of underlying left-sided heart disease, a conservative approach was historically advocated regarding treatment of concomitant TR, assuming that the sole correction of the left-sided heart disease would lead to regression of TR. However, after surgical or transcatheter treatment of mitral regurgitation, aortic stenosis or heart failure treatment, progression or late onset of TR was frequently seen. These insights have led to a paradigm shift in current valvular disease guidelines, advocating a more pre-emptive treatment based on tricuspid annular dilation, regardless of the degree of TR [10]. Other causes leading to pulmonary hypertension include primary pulmonary hypertension, pulmonary embolism and chronic pulmonary disease, similarly leading to increased RV afterload, RV remodelling and dysfunction. In the absence of pulmonary hypertension, intrinsic RV disease, such as arrhythmogenic RV cardiomyopathy or myocardial ischaemia in inferior infarction, can lead to TR through papillary muscle displacement, increased tethering of the TV leaflets and leaflet malcoaptation.
Figure 2. Examples of functional TR (panels A and B) and isolated TR (panels C and D). In a 63-year-old patient two four-chamber apical views are shown in panels A and B. Panel A shows an eccentric mitral regurgitation jet (single arrow), with secondary right atrial, right ventricular and tricuspid annular dilation (double arrow), leading to a moderately severe eccentric TR (panel B), with prolapse of the posterior mitral leaflet (single arrow). Panels C and D show four-chamber apical views of a 73-year-old patient with permanent atrial fibrillation and isolated TR. Note the marked right atrial dilation, with dilation of the tricuspid annulus (double arrow in panel C), leading to a severe TR (panel D), in the absence of RV remodelling.
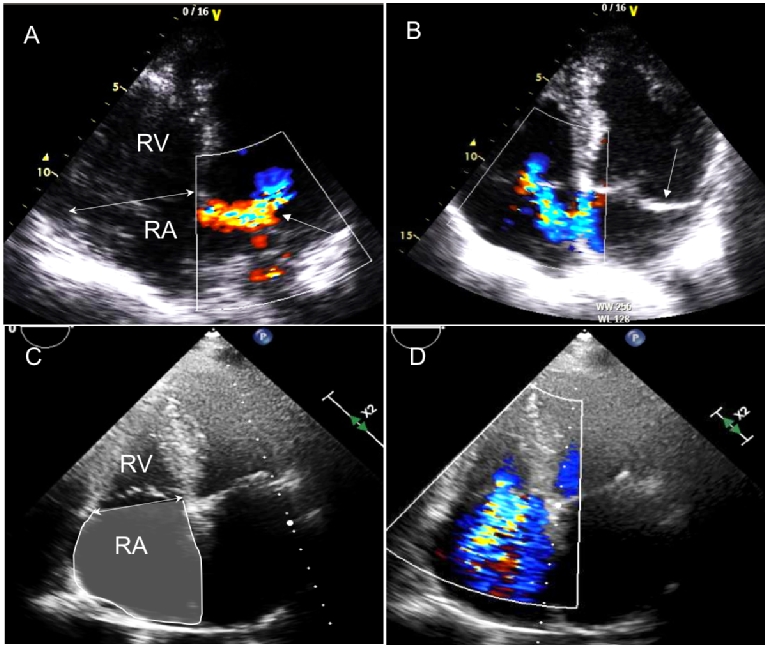
RA: right atrium; RV: right ventricle; TR: tricuspid regurgitation
Isolated TR
Finally, isolated TR is a novel entity most frequently seen in elderly patients with a high prevalence of atrial fibrillation, in the absence of concomitant pulmonary hypertension or co-existing left-sided heart disease. The underlying mechanism is pronounced right atrial and tricuspid annular dilation, leading to leaflet malcoaptation, in the absence of any pronounced RV remodelling [11-13] (Figure 2, panels C and D).
Haemodynamic consequences
TR often remains undetected and is usually coincidentally detected during the work-up for complaints due to co-existing left-sided heart disease. With long-standing significant TR, patients develop signs of right heart failure (ascites, peripheral oedema, weight gain, hepatic dysfunction). On clinical examination, a faint systolic murmur can sometimes be heard; however, often this is inaudible due to the relatively low right-sided pressures. With severe TR, the V-wave of TR merges with the C-wave, forming a single giant C-V-wave, which can be seen in the jugular pulse. In invasive testing, ventricularisation of the right atrial pressure tracing, in which the contour of the right atrial pressure is similar to the contour of the RV pressure, forms the most specific finding but is found in a minority of patients with severe TR [14].
Diagnosing tricuspid regurgitation
A comprehensive understanding of the tricuspid valve morphology, its anatomical variability and the interaction of all its functional components with the valve apparatus (tricuspid annulus, leaflets, chordae tendineae and papillary muscles, RV and atrium) is essential to establish and fine-tune the diagnosis of TR.
Valve anatomy
The tricuspid valve is the largest and most apically positioned of the four cardiac valves and, similar to the mitral valve, its annulus is saddle-shaped, with the lowest part in the posteroseptal position and the highest part in the anteroseptal portion. As the septal part of the tricuspid annulus is more fibrous and fixed to the interventricular septum, tricuspid annular dilation occurs in the anteroposterior direction along the RV free wall. A large variability exists in mobility and length of the three tricuspid leaflets (anterior, posterior and septal), with the anterior usually being the most mobile and longest, and the septal being the least mobile and shortest. The tricuspid leaflets are connected through chordae tendineae with two papillary muscles: the anterior papillary muscle provides chordae to the anterior and posterior leaflets, whereas the posterior provides chordae to the septal and posterior leaflets. Additionally, chordae may arise directly from the RV free wall and moderator band and may promote further leaflet tethering in case of RV dilation.
Grading TR severity by a multiparametric approach
In daily clinical practice, two-dimensional (2D) transthoracic echocardiography remains the cornerstone in establishing the diagnosis of TR and grading its severity by providing qualitative, semi-quantitative and quantitative parameters (Table 1).
Table 1. Qualitative, semi-quantitative and quantitative parameters for diagnosing severe tricuspid regurgitation. Adapted with permission from Lancellotti et al [17].
| Parameters | Severe | Advantages | Limitations |
|---|---|---|---|
| QUALITATIVE | |||
|
- TV morphology |
Flail leaflet, ruptured papillary muscle, severe retraction, large perforation or vegetation. | Direct damage of the tricuspid leaflets is very specific for severe TR | Other abnormalities are non-specific for severe TR |
|
- Flow convergence zone |
Large throughout systole |
Ease of use Good screening for mild vs. severe TR Spatial orientation of the jet |
Inaccurate Influenced by technical and haemodynamic factors Underestimation in eccentric jets adhering to the atrial wall |
|
- CW signal TR jet |
Dense, triangular with early peaking contour (peak <2 m/s in very severe TR) | Simple, easily available | Complete signal difficult to obtain in eccentric jets |
| SEMI-QUANTITATIVE | |||
|
- Colour flow jet area (cm2)
- Colour jet area/RA area (%) |
>10
>33 |
Ease of use Good screening for mild vs. severe TR Spatial orientation of the jet |
Influenced by technical and haemodynamic factors Underestimation in eccentric jets adhering to the atrial wall |
|
- Vena contracta width (cm) |
≥0.7 |
Ease of use Relatively independent of haemodynamic and technical factors |
Not valid for multiple jets Larger errors in small values Influenced by systolic changes in regurgitant flow |
Qualitative parameters include assessment of tricuspid valve morphology, leaflet mobility, colour flow TR jet and continuous wave Doppler spectral signal. Excessive motion of one of the leaflets may point to a traumatic chorda rupture but in case of redundant tissue may also indicate the presence of myxomatous valve disease. Whereas a combination of thickened and restrictive motion of the leaflets may be caused by carcinoid syndrome, the presence of mobile masses attached to the leaflet surface may suggest tricuspid valve endocarditis, as seen in intravenous drug usage. In functional TR, progressive dilatation of the tricuspid valve annulus and RV leads to severe tethering and restrictive motion of morphologically normal leaflets. Exact leaflet identification by 2D echocardiography mostly requires different imaging windows (parasternal, apical and subcostal) and is challenging, as simultaneous visualisation of all three tricuspid leaflets in a single plane is not possible. As the RV and tricuspid valve are positioned anteriorly in the chest, three-dimensional (3D) echocardiography permits improved identification of the three leaflets compared to 2D echocardiography by providing a biplane view or an en face view from the atrial or ventricular side. In lead-induced TR, 3D echocardiography has shown the possibility of identifying the position where impingement of a leaflet occurs and may be used for further safe positioning of the RV pacing lead [15]. Furthermore, a high ratio between the size of the TR colour jet by colour Doppler echocardiography and the right atrial area assessed with colour Doppler echocardiography may indicate the presence of severe TR. However, in case of eccentric regurgitant jets, colour Doppler may severely underestimate the severity of TR (Coanda effect) and therefore should not be used solely for estimation of TR severity. The presence of a dense, triangular signal with early systolic peaking on continuous wave Doppler recordings suggests the presence of severe TR. However, in massive TR, the peak velocity of the regurgitant jet may be paradoxically low due to chronically elevated right atrial filling pressures.
Semi-quantitative parameters are derived from assessment of vena contracta width, proximal isovelocity surface area (PISA) radius, tricuspid valve inflow and hepatic vein flow. In the apical four-chamber view, the vena contracta width is measured in mid systole at the narrowest part of the jet, reflecting the highest regurgitant velocity. A vena contracta width ≥7 mm is indicative of severe TR; however, in case of multiple jets, smaller vena contracta widths can be measured, which does not exclude the diagnosis of severe TR. By adjusting the Nyquist velocity to ±30 cm/sec, the PISA method can be performed in the four-chamber view by measuring the distance from the vena contracta to the first convergence velocity in mid systole, with a PISA radius >9 mm indicative of severe TR. Other semi-quantitative parameters are derived from pulsed-wave Doppler recordings, with a peak velocity of the early diastolic tricuspid inflow (E-velocity) ≥1 m/sec and a systolic reversal of the hepatic vein flow indicative of severe TR.
Finally, by using the flow convergence method, quantification of TR can be performed by calculation of the effective regurgitant orifice area (EROA) and the regurgitant volume (RVol). Cut-offs of an EROA ≥40 mm2 or an RVol ≥45 mL have been shown to carry prognostic importance in patients with isolated TR [16].
By combining the aforementioned parameters, current guidelines advocate a multiparametric approach to improve the diagnostic accuracy of severe TR [17,18] (Table 1).
Right heart evaluation
Evaluation of the haemodynamic impact of TR should be included in every echocardiographic assessment for TR severity by evaluating right atrial and RV dimensions, function, and pressures. From the apical four-chamber view, right atrial short-axis and long-axis dimensions can be assessed and the right atrial area can be measured by tracing the endocardial border at end-systole. Right atrial pressure can be estimated by measuring the inferior vena cava dimensions and respiratory variation in the subcostal view.
Depending on the type of TR, remodelling of the RV and tricuspid annular dilatation may be variably present. In the presence of pulmonary hypertension, the RV remodels in the longitudinal direction (elliptical/spherical deformation), leading to increased valvular tethering with only mild tricuspid annular dilation. In contrast, in isolated TR, in the absence of pulmonary hypertension, the RV tends to dilate predominantly in the basal segments (conical deformation) with more annular dilation and right atrial remodelling. Most frequently, this latter form of RV remodelling occurs in elderly patients with a prior history of atrial fibrillation. By using the modified Bernoulli equation, the pulmonary arterial systolic pressure from the peak TR velocity on continuous wave Doppler recordings can be derived, reflecting the afterload of the RV. Assessment of RV dimensions and function by 2D echocardiography is challenging owing to its complex crescent-shaped geometry [19]. Although they are load-dependent and measure only a singular segment of a complex 3D structure, myocardial velocities and tricuspid annular plane systolic excursion (TAPSE) (<17 mm is considered abnormal) are the most commonly used markers of RV function. Fractional area change expresses the percentage of change in RV area between end-diastole and end-systole; a value <35% is considered abnormal. Less angle and load dependent, RV myocardial longitudinal strain by 2D speckle tracking echocardiography is calculated as the percentage of systolic shortening of the RV free wall from base to apex measured in the RV-focused apical four-chamber view. Reference values for global RV strain corrected for sex have been reported; an RV global longitudinal strain (GLS) of the free wall ≥20% is likely to be abnormal [20].
Three-dimensional imaging modalities, such as cardiovascular magnetic resonance imaging (CMR) and 3D echocardiography, directly measure RV volumes without the need for geometric assumptions and are considered more accurate than 2D echocardiography. CMR is currently considered the standard reference technique due to its unique capability in providing accurate and reproducible assessment of function and tissue characterisation.
Conclusion
The tricuspid valve was once deemed the forgotten valve. However, insights into the epidemiology and aetiology of tricuspid regurgitation have grown vastly over the last decade. The advent of advanced imaging techniques has provided novel insights into the important role of right heart remodelling in the pathophysiology of TR which may lead to improved risk stratification and more timely intervention in the near future.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.