Background
The pericardium, derived from the Greek words περί, 'around', and κάρδιον, 'heart', is a thin fibroelastic sac which contains the heart and roots of the great vessels. It consists of two layers: a serous visceral (inner portion) and a fibrous parietal layer (outer portion). In physiologic conditions, the pericardial cavity contains 10 mL to 50 mL of plasma ultrafiltrate (i.e. pericardial fluid). [1] Inflammation of the pericardial sac is called pericarditis.
The main pericardial syndromes encompass pericarditis (acute, subacute, chronic, recurrent), pericardial effusion, cardiac tamponade and pericardial masses. Pericarditis is the most common form of pericardial disease worldwide and is typically encountered in young and middle-aged people. [2] It represents 0.2% of all hospital admissions of cardiovascular aetiology and approximately 5% of patients with nonischaemic aetiology chest pain, presenting in the emergency departments of North America and Western Europe. [2] Acute pericarditis is the most common pericardial syndrome in clinical practice. [3]
This article reviews the causes, clinical picture, inflammatory parameters, electrocardiogram (ECG) and multimodality imaging findings and briefly discusses the differential diagnosis of acute pericarditis.
Aetiology
Pericarditis may be an isolated disease or the first manifestation of an underlying systemic disease. Causes of acute pericarditis can be broadly classified into infectious and non-infectious (Table 1). [3,4] The aetiology is multifactorial and depends on the epidemiological background, patient population and clinical setting. [3] In particular, the most common cause of pericarditis in developed countries is viruses, whereas tuberculosis is the most frequent cause in developing countries. With regard to endemic regions, tuberculosis often coexists with human immunodeficiency virus (HIV). Notably, the most common noninfectious causes are secondary to autoimmune diseases, metastatic tumours and post-cardiac injury syndrome. [5]
Table 1. Main Causes of Acute Pericarditis [1-3,6,19]
|
Categories |
A. Idiopathic |
|
Causes |
Unknown |
|
Frequency |
Most frequent cause |
|
Categories |
B. Infectious Causes |
|
Causes |
|
|
Frequency |
|
|
Categories |
Viral |
|
Causes |
Epstein-Barr, influenza, hepatitis, human immunodeficiency virus, mumps, echovirus, adenovirus, cytomegalovirus, varicella, rubella, human herpesvirus, parvovirus, coxsackie |
|
Frequency |
Most frequent cause in developed countries |
|
Categories |
Bacterial |
|
Causes |
Mycobacterium tuberculosis, Coxiella burnetii, streptococcus, staphylococcus, pneumococcus, legionella, salmonella, haemophilus |
|
Frequency |
Rare (with the exception of mycobacterium tuberculosis) |
|
Categories |
Fungal |
|
Causes |
Candida, aspergillosis, histoplasmosis, blastomycosis |
|
Frequency |
Very rare |
|
Categories |
Parasitic |
|
Causes |
Toxoplasma, echinococcus |
|
Frequency |
Very rare |
|
Categories |
C. Non-Infectious Causes |
|
Causes |
|
|
Frequency |
|
|
Categories |
Neoplastic |
|
Causes |
Primary: pericardial mesothelioma Secondary tumours: leukemia, breast cancer, lung cancer, lymphoma, melanoma |
|
Frequency |
Frequent as secondary metastasis |
|
Categories |
Metabolic |
|
Causes |
Hypothyroidism, renal failure, hypercholesterolaemia, gout, anorexia nervosa |
|
Frequency |
Frequent |
|
Categories |
Cardiovascular |
|
Causes |
Acute myocardial infarction, Dressler's syndrome, aortic dissection |
|
Frequency |
Frequent |
|
Categories |
Autoimmune |
|
Causes |
Rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, dermatomyositis, sarcoidosis, systemic vasculitides, Behçet's syndrome, familial Mediterranean fever |
|
Frequency |
Frequent |
|
Categories |
Traumatic and iatrogenic |
|
Causes |
Catheterisation, surgery, chest trauma, radiation |
|
Frequency |
Frequent |
|
Categories |
Drug-related |
|
Causes |
Phenytoin, minoxidil, isoniazid, procainamide, hydralazine, methyldopa, doxorubicin, amiodarone, clozapine, streptomycin |
|
Frequency |
Rare |
|
Categories |
Other |
|
Causes |
Congenital absence of pericardium |
|
Frequency |
Rare |
|
Categories |
Causes |
Frequency |
|---|---|---|
|
A. Idiopathic |
Unknown |
Most frequent cause |
|
B. Infectious Causes |
|
|
|
Viral |
Epstein-Barr, influenza, hepatitis, human immunodeficiency virus, mumps, echovirus, adenovirus, cytomegalovirus, varicella, rubella, human herpesvirus, parvovirus, coxsackie |
Most frequent cause in developed countries |
|
Bacterial |
Mycobacterium tuberculosis, Coxiella burnetii, streptococcus, staphylococcus, pneumococcus, legionella, salmonella, haemophilus |
Rare (with the exception of mycobacterium tuberculosis) |
|
Fungal |
Candida, aspergillosis, histoplasmosis, blastomycosis |
Very rare |
|
Parasitic |
Toxoplasma, echinococcus |
Very rare |
|
C. Non-Infectious Causes |
|
|
|
Neoplastic |
Primary: pericardial mesothelioma Secondary tumours: leukemia, breast cancer, lung cancer, lymphoma, melanoma |
Frequent as secondary metastasis |
|
Metabolic |
Hypothyroidism, renal failure, hypercholesterolaemia, gout, anorexia nervosa |
Frequent |
|
Cardiovascular |
Acute myocardial infarction, Dressler's syndrome, aortic dissection |
Frequent |
|
Autoimmune |
Rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, dermatomyositis, sarcoidosis, systemic vasculitides, Behçet's syndrome, familial Mediterranean fever |
Frequent |
|
Traumatic and iatrogenic |
Catheterisation, surgery, chest trauma, radiation |
Frequent |
|
Drug-related |
Phenytoin, minoxidil, isoniazid, procainamide, hydralazine, methyldopa, doxorubicin, amiodarone, clozapine, streptomycin |
Rare |
|
Other |
Congenital absence of pericardium |
Rare |
Diagnostic evaluation
The main diagnostic evaluation consists of medical history (recent viral infection) and physical examination (to detect pericardial rubs at auscultation and additional possible signs of a systemic disease that may be responsible for pericarditis); blood tests (WBCs, ESR, CRP, troponin, complete blood count [CBC], urea, creatinine); ECG; transthoracic echocardiography (TTE); and chest X-ray.
Clinical presentation
Acute pericarditis is defined as an 'inflammatory pericardial syndrome with or without pericardial effusion'. [3] The diagnosis is clinical and can be made based on two of the following criteria: a) pericardial chest pain in the patient’s medical history b) pericardial rubs upon auscultation c) new widespread ST-elevation or PR depression on ECG d) pericardial effusion (new or worsening). Supportive findings are elevation of inflammatory markers and evidence of pericardial inflammation by computed tomography (CT) or cardiac magnetic resonance (CMR). [3] Specific features at presentation such as temperature >38°C, subacute onset, large pericardial effusion (>20 mm) or tamponade and non-response to aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) are major factors of poor prognosis. Minor factors include concomitant myocarditis, immunodepression, trauma and oral anticoagulant therapy. [3]
Patients with acute pericarditis typically present with chest pain (>85% to 90% of cases) that usually radiates to the trapezius ridge of the left shoulder or arm and resembles ischaemic pain. However, retrosternal pain in acute pericarditis is mainly sharp and pleuritic, which augments in the supine position with cough and deep inspiration. Conversely, it usually subsides in the upright position and by leaning forward due to decrease of the pressure on the parietal pericardium. [6] Common associated signs and symptoms include low-grade intermittent fever, dyspnoea, cough, malaise, myalgia and occasionally hiccoughs. The medical history often reveals symptoms suggestive of viral infection.
Auscultation exposes a pericardial friction rub (pathognomonic sign for pericarditis) due to increased friction of inflamed pericardial layers in about one-third of patients with acute pericarditis. It is a superficial scratchy or squeaking sound, best heard with the diaphragm of the stethoscope over the left sternal border, with the patient leaning forward. It typically consists of three phases corresponding to movement of the heart during atrial systole (absent in atrial fibrillation), ventricular systole, and the rapid filling phase of early ventricular diastole. [2,6] Sometimes pericardial rub has only two components or even one component. Notably, pericardial friction rub can disappear not only at healing of pericarditis, but also when there is development of more pericardial fluid in the pericardial sac, reflecting either improvement or exacerbation of the disease. In addition, friction rub in acute pericarditis can vary in intensity from moment to moment, which rarely happens with other forms of pericarditis. The auscultation of pericardial friction rub is highly specific for acute pericarditis (specificity approximately 100%).
ECG findings
Typical ECG findings in acute pericarditis are present in no more than 60% of cases and evolve in four stages (Figure 1). In particular, ECG initially (within hours to days) depicts diffuse concave ST-segment elevation (in all leads, except aVR and often V1) with concomitant PR depression (first stage), followed by (within the first week) return to baseline of the latter deviations and T-wave flattening (second stage), diffuse T-wave inversion after the ST-segment has become isoelectric (third stage) and, finally, ECG normalisation or persistence of T-wave inversions (fourth stage). [6-8] Sustained atrial or ventricular arrhythmias are not frequent in acute pericarditis, and their presence denotes concomitant myocarditis or another not-relevant cardiac disease. [9]
Figure 1. ECG of a 32-Year-Old Man with Acute Pericarditis, Presenting with Chest Pain (Note the characteristic concave ST elevation in leads I, II, aVL and V5-V6.)
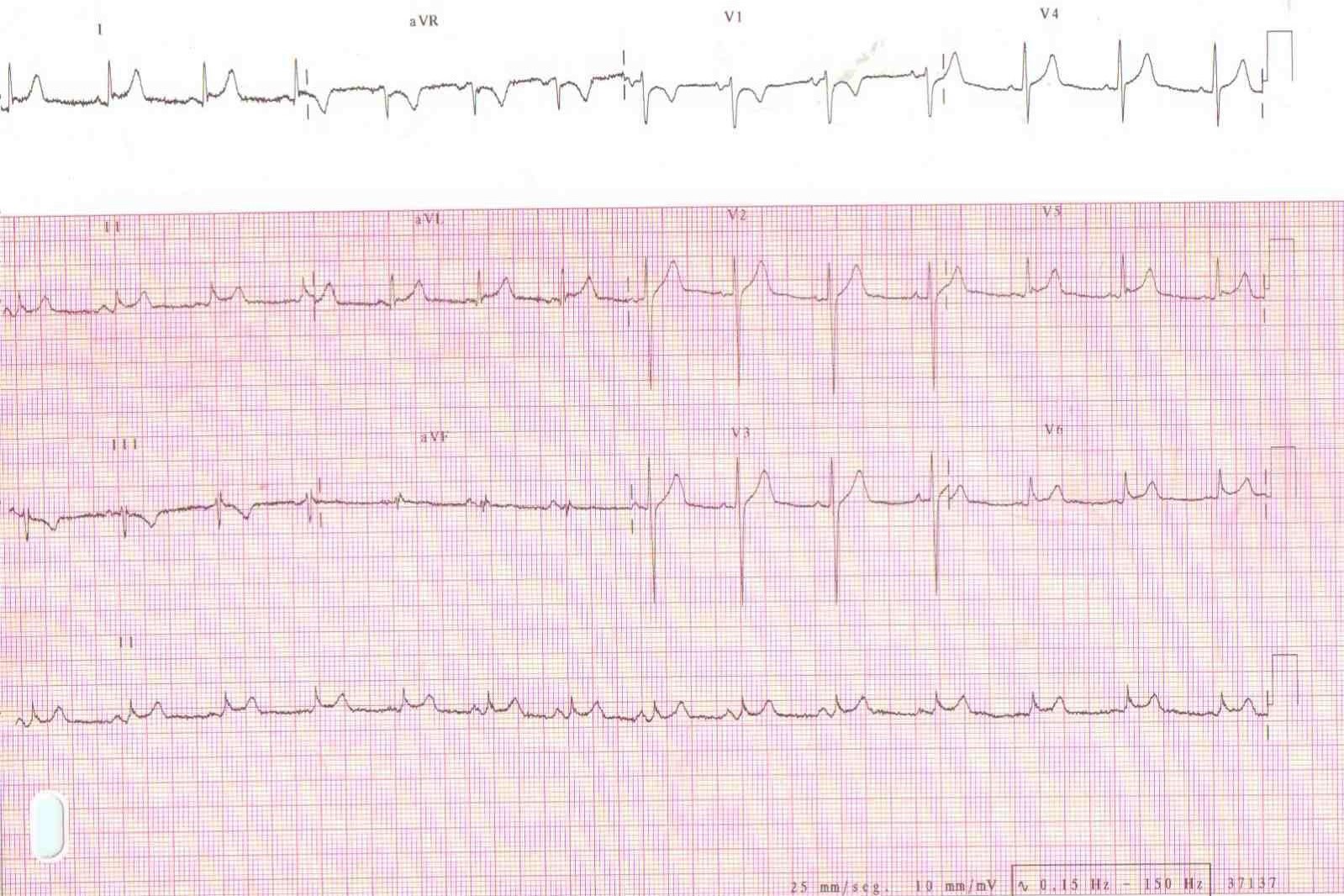
The ECG of acute pericarditis often resembles that of ST-elevation myocardial infarction (STEMI). ECG findings suggestive of acute pericarditis are: a) the occurrence of ST-elevation less than 5 mm b) ST-segment concavity c) more extensive lead involvement d) less prominent reciprocal ST-segment depression e) PR-segment elevation in aVR, with reciprocal PR-segment depression in other leads f) the absence of abnormal Q-waves g) variability in the time of T-wave inversion occurrence following ST-segment elevation h) the lack of QRS widening and QT interval shortening in leads with ST elevation. [10]
Sometimes, acute pericarditis ECG should be differentiated from that of early repolarisation. Notably, in acute pericarditis, the ratio of ST elevation to T-wave amplitude in lead V6 usually exceeds 0.24 (positive and negative predictive values are both 100%). This ECG sign is typical for the differential diagnosis with early repolarisation. [11]
An interesting study proposed the use of leads I and V4-V6 in order to differentiate acute pericarditis from early repolarisation and left ventricular hypertrophy. [12] In particular, the authors concluded that a ratio of the ST amplitude segment to the amplitude of the T-wave ≥0.25, in leads I, V4, V5 and V6, was predictive of acute pericarditis. Notably, the best predictive value of the ST/T ratio appeared when ST elevation presented in lead I.
Laboratory tests - markers of inflammation
Elevation of inflammatory markers is common in patients presenting with acute pericarditis. Among them, the most widely used are the white blood cells (WBCs), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). CRP is used not only to confirm the suspicion of acute pericarditis, but also to monitor the duration of disease activity and regulate the treatment based on the individual patient’s characteristics. [13]
In the presence of concomitant myocarditis and pericarditis (myopericarditis), serum biomarkers of myocardial injury, such as CK and troponin I or T, are increased. The evaluation of troponins is mainly for the exclusion of myopericarditis because they do not seem to exhibit prognostic value on acute pericarditis. [14] Interestingly, pericarditis patients with elevated biomarkers of myocardial injury almost always exhibit ECG changes characteristic of ST-segment elevation.
The prognostic role is promising for carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1), which is an immune inhibitory protein, and for MHC class I chain related protein A (MICA), which exhibits an immune stimulating function in patients with acute and recurrent pericarditis. [15] CEACAM1 and MICA are serum proteins that can be released from damaged or inflamed tissues in patients with pericarditis. Markel et al. [15] reported that patients with acute pericarditis show high serum CEACAM1 levels, either due to unique splicing in the injured pericardial cells or due to other mechanisms, such as proteolytic cleavage. In the same study, serum MICA levels were elevated in some of the pericarditis patients, but without reaching statistical significance. However, in the subgroup of patients with recurrent pericarditis, a significant positive correlation between serum MICA concentrations and recurrences was observed independently of other potentially confounding parameters linked to recurrences. [15] The authors concluded that CEACAM1 could serve as a potentially novel biomarker for pericarditis, and MICA as an innovative prognostic marker in these patients.
A routine laboratory evaluation should include markers of renal function (urea, creatinine), electrolytes, LDH and transaminases (SGOT, SGPT). If there is a clinical indication, more specific tests may be ordered, such as blood cultures, thyroid function hormones, antinuclear antibodies (ANA), anti-neutrophil cytoplasm antibodies (ANCA), anti-extractable nuclear antigens (ENA), HIV testing and even pericardial biopsy. [3]
Chest X-ray
In patients presenting with acute pericarditis, the chest X-ray is usually within normal limits since an increased cardiothoracic index is exhibited when pericardial effusion is more than 300 mL. Signs of pleuropericardial involvement may be found in the background of pleuropulmonary disease in the chest X-rays of patients with acute pericarditis. Chest X-rays may reveal malignancies, sarcoidosis, or several infections, which may be the cause of acute pericarditis. Occasionally, small pleural effusions are present, presumably due to viral or possibly mycoplasmal infections. A patient presenting with new and otherwise unexplained cardiomegaly, especially in the setting of clear lung fields, should be evaluated for acute pericarditis. [6]
Echocardiography
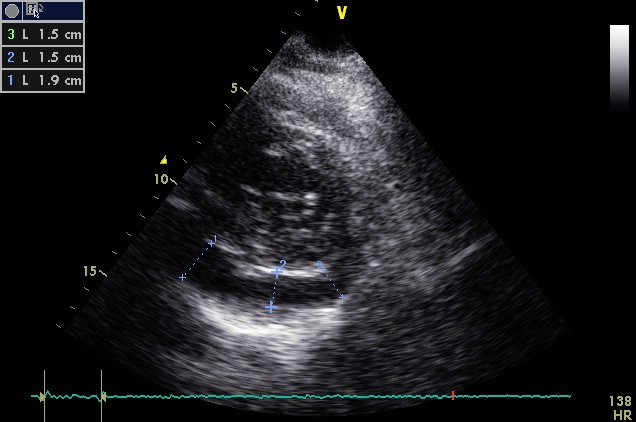
Echocardiography is low cost, widely available and can be performed on an urgent basis. It provides valuable information regarding the thickening and hyperechogenicity of pericardial layers, the presence or absence of pericardial effusion, its volume and its concomitant haemodynamic effects (tamponade, restriction). [6,16] Pericardial effusion, either new appearing or worsening, is typically mild and is evident in 60% of cases (Figure 2). [6] With the use of the M-mode, pericardial effusion is demonstrated as an echo-free space between the inner and outer layer of pericardium at cardiac systole and diastole, whereas a simple systolic separation may be considered physiologic. [1] Pericardial effusion, based on the size at diastole, is characterised as mild (<10 mm), moderate (10-20 mm), or large (>20 mm). [1] Unfortunately, TTE may exhibit a number of limitations, such as poor acoustic window due to obesity or chronic obstructive pulmonary disease (COPD), limited tissue characterisation, and a relatively high dependence on the operator. [17] That is why several other imaging techniques have evolved during recent years assisting in the diagnostic workup of patients with pericarditis.
Figure 2. The Echocardiographic Examination (parasternal short-axis view) of a 60-Year-Old Woman with Acute Pericarditis Reveals Echo-Free Space (>1 and <2 cm) Located Posteriorly, Suggestive of Moderate Pericardial Effusion
CT/CMR
Multimodality imaging is an integral part of an acute pericarditis diagnostic workup and, apart from echocardiography which is considered the first-line test, includes CT and/or CMR (second-line imaging tests). Cardiac CT manifests several advantages regarding pericarditis investigation, such as the assessment of pericardial calcifications and thickening and also the evaluation of concomitant diseases. Advanced techniques such as ECG gating provide excellent images without motion artefacts. [18] However, major limitations are the use of ionising radiation and iodinated contrast, the unmet need for breath-hold and the inability to assess unstable patients or patients with arrhythmias. [6] On the other hand, CMR can provide the characterisation of cardiac tissue, the assessment of thickening and constriction, and the evaluation of myocardial involvement. CMR does not require radiation or nephrotoxic contrast agents. Furthermore, baseline late gadolinium enhancement (LGE) seen on CMR may correlate with inflammation severity. [18] A CMR protocol for pericardial disease usually encompasses cine imaging, black-blood imaging of the heart and pericardium, tagging, phase-contrast sequences and delayed enhancement imaging in order to evaluate the LGE. [18] Unfortunately, this technique also has several weaknesses as it is time-consuming, high cost, not widely available, and requires stable patients and rhythms. In addition, patients with severe renal impairment and pacemakers may not be eligible candidates. [6]
Differential diagnosis
A number of pathologies characterised by chest pain, such as acute coronary syndromes, aortic dissection, pulmonary embolism, pneumonia, pneumonitis, gastric ulcer, gastroesophageal reflux disease, pneumothorax and herpes zoster, are part of the differential diagnosis with acute pericarditis (Table 2). [19,20]
Table 2. Differential Diagnosis of Acute Pericarditis [19,20]
Angina |
Acute coronary syndromes |
Angina |
Aortic dissection |
Angina |
Aortic stenosis |
Angina |
Oesophagitis |
Angina |
Oesophageal spasm |
Angina |
Oesophageal rupture |
Angina |
Gastric ulcer |
Angina |
Pancreatitis |
Angina |
Pulmonary embolism |
Angina |
Pulmonary hypertension |
Angina |
Pneumonia |
Angina |
Pneumonitis |
Angina |
Pleuritis |
Angina |
Tuberculosis |
Angina |
Pneumothorax |
Angina |
Musculoskeletal disorders |
Angina |
Trauma |
Angina |
Herpes zoster |
Angina |
Depression |
Angina |
Panic disorder |
Angina |
|---|
|
Acute coronary syndromes |
|
Aortic dissection |
|
Aortic stenosis |
|
Oesophagitis |
|
Oesophageal spasm |
|
Oesophageal rupture |
|
Gastric ulcer |
|
Pancreatitis |
|
Pulmonary embolism |
|
Pulmonary hypertension |
|
Pneumonia |
|
Pneumonitis |
|
Pleuritis |
|
Tuberculosis |
|
Pneumothorax |
|
Musculoskeletal disorders |
|
Trauma |
|
Herpes zoster |
|
Depression |
|
Panic disorder |
Acute pericarditis is usually distinguished from myocardial ischaemia or infarction (based on the clinical findings, ECG, markers of myocardial necrosis and imaging modalities such as echocardiography), but coronary angiography is sometimes required to resolve the issue. Finally, not infrequently, acute pericarditis is a manifestation of a presenting, silent MI.
Specific forms of pericarditis
According to the 2015 ESC guidelines on pericardial disease [3], the definite diagnosis of viral pericarditis derives from the comprehensive workup of histological, cytological, immunohistological and molecular investigations in pericardial fluid and pericardial/epicardial biopsies; otherwise the term 'presumed viral pericarditis' should be used. For the diagnosis of purulent pericarditis, urgent pericardiocentesis is recommended and pericardial fluid should be sent for bacterial, fungal and tuberculous studies. And blood should be drawn for cultures. Furthermore, urgent TTE or CT is recommended in patients with a history of chest trauma and systemic arterial hypotension. Lastly, as far as malignancies are concerned, cytological analyses of pericardial fluid are recommended.
Conclusions
Acute pericarditis is the most frequent among the pericardial syndromes. Its aetiology is multifactorial. The cardinal symptom is chest pain that sometimes needs to be differentiated from the ischaemic pain. Diagnosis is clinical and is made when two out of the four following criteria are fulfilled: a) chest pain b) pericardial rubs c) ECG changes d) pericardial effusion. Supportive findings are the increase in inflammatory markers and evidence of inflammation in imaging (CT/CMR). The emerging role of new inflammatory markers (i.e. CEACAM1 and MICA) is hopeful. Multimodality imaging is vital during the diagnostic workup of patients with acute pericarditis.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.