Introduction
There are 10-14 million patients with heart failure and 1-2 million patients with end-stage chronic heart failure (ESHF) in Europe. ESHF has one of the largest effects on quality of life of any advanced disease with an average survival of less than one year [1]. Until recently, there was little structural support for these patients, to extend life, and to improve functional status. However, advances in heart transplantation (HTx), improved mechanical assist devices, and novel, multidisciplinary and palliative approaches have all contributed substantially to better care of this growing patient population. Timely referral of eligible patients for advanced therapies is critical for an optimal outcome [2].
Patients with ESHF are defined as being in NYHA Class III-IV heart failure despite optimal medical and device therapy (Seattle Heart Failure Score 2-3). The 6-minute walk is usually <300 m and the peak oxygen consumption <12 ml/kg/min [1,2]. Often an intolerance to or withdrawal of evidence-based heart failure medications (e.g., beta-blockers, ACE inhibitors, aldosterone antagonists) due to hypotension is present. Signs of secondary organ dysfunction, such as renal and/or hepatic dysfunction with volume overload, are also typically present. Frequent HF hospitalisations and the intermittent application of intravenous inotropes are frequently necessary.
Who should be referred to a heart failure centre?
Death in ESHF patients is primarily related to heart failure and its comorbidities and may therefore be served by advanced heart failure therapy.
The Seattle Heart Failure Model (www.depts.washington.edu/shfm) is one of the most commonly used multivariable instruments for estimating prognosis in ESHF and includes NYHA function class, ischaemic aetiology, diuretic dose, left ventricular ejection fraction, systolic blood pressure, sodium, haemoglobin, percent lymphocytes, uric acid, and cholesterol [1,3]. Rounded scores from 0-4 are calculated, with the highest score describing a two-year mortality rate of 11% [3]. A patient with a score ≥2 should be seen at least annually by a heart failure specialist for optimised medical and/or interventional therapy and potential evaluation for HTx, assist device therapy or palliative care.
Based on a recent Swedish study [4], risk factors for survival in ESHF patients <80 years are: systolic blood pressure ≤90 mmHg, creatinine ≥160 µmol/l, haemoglobin ≤120 g/l, no renin-angiotensin system antagonist, no beta-blocker. The presence of 1, 2, or 3-5 of these risk factors is associated with a one-year mortality of 21%, 40%, and 61%, respectively [4]. The presence of one or more risk factors should be a trigger for generalists to refer patients to an advanced heart failure centre [4].
Therapeutic options in ESHF patients include evidence-based pharmacotherapy, fluid removal, intermittent inotropes and palliative care. Other interventional options include electrical therapies, treatment of valvular and ischaemic diseases, mechanical assist device implantation, and HTx (Figure 1).
Figure 1. Therapeutic options in ESHF patients.
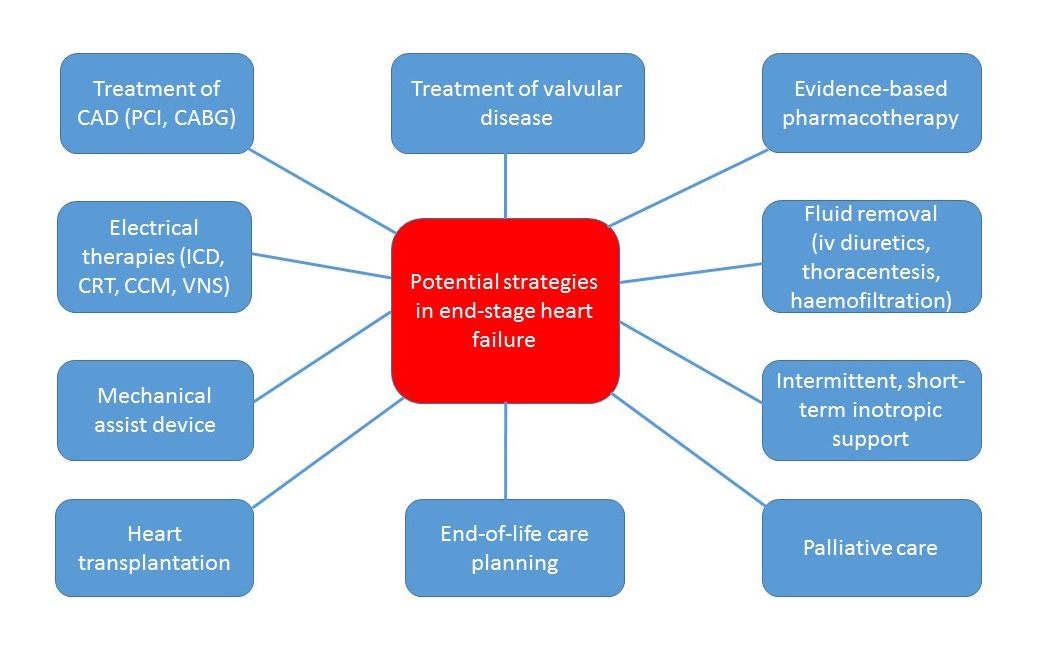
CABG: coronary artery bypass grafting; CAD: coronary artery disease; CCM: cardiac contractility modulation; CRT: cardiac resynchronisation therapy; ICD: implantable cardioverter defibrillator; PCI: percutaneous coronary intervention; VNS: vagus nerve stimulation
Transplantation
HTx is an accepted treatment for ESHF and provides significantly increased survival, exercise capacity, quality of life and return to work compared with conventional treatment [5].
The 2016 International Society for Heart Lung Transplantation (ISHLT) updated criteria for HTx are as follows [6]: an estimated one-year survival of <80% (in patients ≤65 years), as calculated by the Seattle Heart Failure Model, should be considered as a reasonable cut point for listing; specific indications include an ejection fraction less than 20%, intractable angina or malignant cardiac arrhythmias for which conventional therapy has been exhausted.
Relative contraindications include a pre-transplant body mass index (BMI) >35 kg/m2, renal dysfunction with a GFR <30 ml/min/1.73 m2, pulmonary vascular resistance >3 Wood units and patients for whom social supports are deemed insufficient to achieve compliant care in the outpatient setting [6].
The use of mechanical circulatory support should be considered for patients with potentially reversible or treatable comorbidities, such as cancer, obesity, renal failure, tobacco use, and pharmacologically irreversible pulmonary hypertension, with subsequent re-evaluation to establish candidacy.
The one-year survival rate after HTx is as high as 82%, with a five-year survival rate of 70% (ISHLT registry). A significant number of recipients survive more than 10 years after the procedure [6].
In the Eurotransplant region (ET; Austria, Belgium, Germany, Luxembourg, The Netherlands, and Slovenia), an urgency-based donor heart allocation system was adopted in 2000, giving the highest priority for HTx to patients with the highest risk of dying. High-urgency transplantation is needed in those patients who deteriorate rapidly and develop acute-to chronic heart failure or severe end-organ dysfunction. Currently, nearly 1,000 patients with end-stage heart failure are listed for HTx in Germany. All patients with ET high-urgency status have to be treated in intensive care units and have to be relisted every eight weeks. The mean waiting time for these patients is about six months and sometimes exceeds one year. Haemodynamic stabilisation within an intensive care unit for several weeks or months, including avoidance of severe kidney and/or liver dysfunction, is a tremendous challenge.
Approximately 75% of transplant recipients require inotropic or mechanical circulatory support at the time of HTx. Since in the last 15 years much sicker patients have been transplanted compared to before 2000, survival in the first five years post-transplant has decreased over time in the ET region. In general, the sicker the patient is pre-transplant, the worse the survival – underscoring the importance of a new allocation system that considers the medical status before transplantation and the potential survival in order to optimise outcomes. HTx should be reserved for those patients in whom the maximum clinical benefit can be expected, such as younger patients with few comorbidities.
Unfortunately, the question of recipient selection and listing status continues to pose medical and ethical dilemmas.
Ventricular assist devices
Screening for potential left ventricular assist device (LVAD) candidates should be performed in advance in ESHF patients.
LVADs improve quality of life and survival in ESHF. LVADs are initially used mainly as a bridge to HTx; however, with the emerging donor-organ shortage and the improved outcome using continuous flow devices, they are increasingly used as a destination therapy [7].
LVAD technology has seen significant improvements since its inception. Today, patients with LVADs as destination therapy have survival outcomes that rival LVADs as bridge to transplantation therapy. With the development of new technologies resulting in smaller, more durable, and improved biocompatible devices, LVAD therapy has the potential for increased longevity and widespread application for patients with advanced heart failure.
The criteria for LVAD implantation are NYHA Class IV heart failure refractory to optimal medical therapy, LVEF less than 25%, systolic blood pressure <80 mmHg, pulmonary capillary wedge pressure >20 mmHg, cardiac index <2.0 l/min/m2 despite continuous intravenous inotropic therapy and intra-aortic counterpulsation [7,8].
With third-generation LVADs (e.g., HeartWare; HeartWare International Inc., Framingham, MA, USA, or HeartMate 3; St. Jude Medical, St. Paul, MN, USA), rates of survival and freedom from adverse events are still increasing. Survival rates are about 90% at six months, 85% at one year and about 75% at two to three years after implantation, respectively [8]. Biventricular assist devices in patients with severe right and left ventricular dysfunction have less favourable outcomes compared to patients with LVADs
The main complications of recent LVADs include re-exploration rates for bleeding (14%), driveline infections (10%), gastrointestinal bleeding (8%) and stroke (8%). Age, comorbidities, right ventricular function and pulmonary vascular resistance are the main prognostic factors for patients with LVADs [8].
After LVAD, re-evaluation of haemodynamics should be carried out in transplant candidates after three to six months to ascertain reversibility of pulmonary hypertension [6].
Life-threatening assist device complications such as severe infections, repeated assist-related cerebral events, or severe recurrent ventricular rhythm disorders in patients ≤65 years may allow high-urgent listing for HTx in the ET region.
Inotrope therapy
End-stage heart failure patients may develop symptoms that are refractory to oral therapies. Current management guidelines recommend consideration of intravenous inotrope (dobutamine, noradrenaline, levosimendan) therapy for palliation of symptoms in ESHF patients [9]. Although meta-analyses have reported that mortality is not reduced with the administration of inotropes [10,11], inotropes can reduce hospitalisations and improve functional status, and thus are often used as a pharmacologic bridge to HTx or mechanical support, or as part of palliative care [2,12].
However, long-term continuous inotrope treatment is associated with tachyphylaxia, arrhythmias and increased mortality [13,14]. Based on the constantly increasing waiting times for high-risk HTx patients (sometimes exceeding 12 months), a modulated, individualised inotropic regimen is necessary [15-17]; however, sufficient prospective randomised studies are lacking.
Recent publications demonstrate that intermittent levosimendan has resulted in better survival and/or haemodynamic benefits when compared to a regimen consisting of intermittent dobutamine infusions or placebo [16,18]. One of the major advantages of levosimendan may be its longer half-life and less pronounced pro-arrhythmic effects. Indeed, after a 24-hr infusion of levosimendan, its pharmacodynamic effects persist for at least one week [19] with haemodynamic and neurohumoral effects detectable even after four weeks [20].
Importantly, inotrope dependence, defined as clinical deterioration after inotrope infusion withdrawal, indicates a further increase in risk with a median survival of <6 months [17].
Palliative care
Palliative care for ESHF should be integrated into comprehensive ESHF care. The neurohormonal and catabolic derangements in ESHF are the base of heart failure symptoms [21]. Recent studies have shown that pain, fatigue, breathlessness, anxiety, and depression are the most frequent symptoms of the patients with terminal illnesses [21,22]. Differently from patients with cancer, patients with ESHF generally have important comorbidities that further worsen quality of life and that may dominate patients’ clinical problems.
Both potential sudden cardiac death and generally shortened length of life by ESHF should be acknowledged and planned for. Palliative care in ESHF includes a focus on symptom relief, emotional support and communication between patient and her/his family. Potential management providing palliation of symptoms includes: morphine for reducing breathlessness, pain and anxiety; increasing inspired oxygen concentration to provide relief of dyspnoea; diuretics to relieve severe congestion; reducing HF drugs to maintain cerebral and second organ blood flow and to reduce the risk of falls [5].
Recent technological advances have led to the use of defibrillators, resynchronisation pacemakers and assist devices as viable treatment options for patients with ESHF. This has led to increased complexity of care and decision making at the end of life. As a result, advanced care planning for patients with ESHF must be addressed earlier in the course of the disease and before the end of life, which would allow patients the opportunity to review the issues surrounding death from heart failure, before the development of an acute exacerbation [2,5,21].
Patients with ESHF are willing to address their end-of-life preferences, often valuing longevity even at older age, but individual preferences are impossible to predict and may change over time, reinforcing the value of listening to patients to provide relevant insight and individualise care [22]. Openness and communication about prognosis, and realistic treatment possibilities engender hope and allow patients to plan for their future [22].
Conclusion
The importance of shared decision making in ESHF cannot be overstated, given the complex variety of treatment options. Evaluation for heart transplantation and/or assist device therapy should be performed at an early stage of the progressive disease [1]. An annual heart failure review visit should include discussion of current and potential therapies. The discussion should include outcomes beyond survival, including major adverse events, symptom burden, functional limitations and quality of life. Cardiologists should take responsibility for initiating the development of a comprehensive plan for end-of-life care consistent with patient values, preferences and goals [1].


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.