Take-home messages
- Early referral for assessment and treatment of TR is essential.
- Transcatheter techniques are safe and efficient in reducing TR and improving symptoms of heart failure.
- Multiple transcatheter techniques are available for TR treatment and appropriate device selection based on tricuspid valve anatomy is necessary for optimal procedural results.
- Currently ongoing randomised control trials will show the impact of these procedures on life expectancy and clinical outcomes.
TR treatment strategies – general considerations
Guideline-directed medical therapy is the cornerstone and first step of tricuspid regurgitation (TR) treatment, along with addressing comorbidities, such as left-sided valvular diseases, coronary artery disease, cardiomyopathy, pulmonary diseases, or atrial fibrillation [1]. Diuretics are particularly effective in reducing systemic volume overload and alleviating symptoms related to right heart failure. However, when TR progresses or heart failure symptoms persist despite the optimal medical therapy, interventional strategies for TR treatment should be considered – especially in case of secondary TR associated with progressive heart failure with unfavourable outcomes. Interdisciplinary discussion within an experienced Heart Team is crucial.
Surgical tricuspid valve (TV) reconstruction is generally preferred over replacement and minimally invasive beating-heart endoscopic techniques are rapidly developing [2].
Transcatheter tricuspid valve interventions
Transcatheter TV interventions have emerged as a promising alternative for patients with isolated TR who are at high surgical risk. Early experience of transcatheter techniques in Europe have demonstrated the safety and feasibility of reducing TR [3], with improvements in heart failure symptoms and quality of life. Furthermore, a propensity score-matching analysis has shown the potential benefit of transcatheter tricuspid valve intervention on long-term survival compared to medical therapy [4]. The European Society of Cardiology (ESC) guidelines recommend transcatheter treatments in symptomatic patients with isolated secondary TR who are considered inoperable by the Heart Team discussion [1]. Currently, multiple transcatheter devices are commercially available in the EU (Figure 1), and several are under investigation (Figure 2). Proper device selection is key to a successful intervention and postinterventional results (Figure 3).
Figure 1. CE-marked devices for transcatheter tricuspid valve intervention.
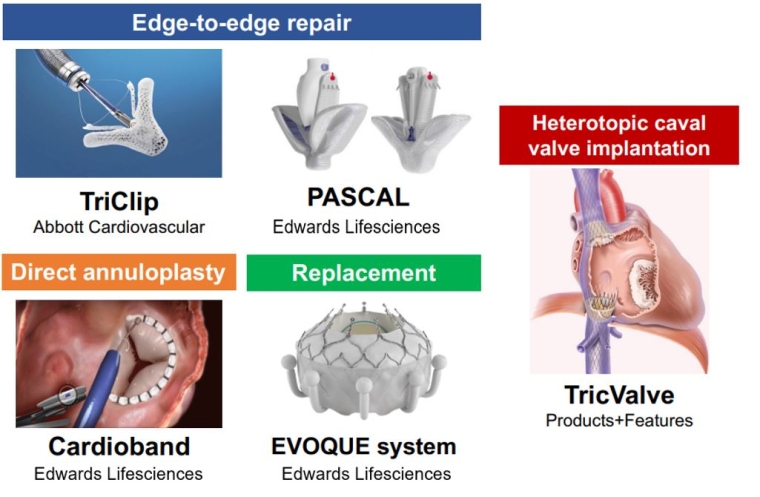
Figure 2. Representative transcatheter devices under investigation.
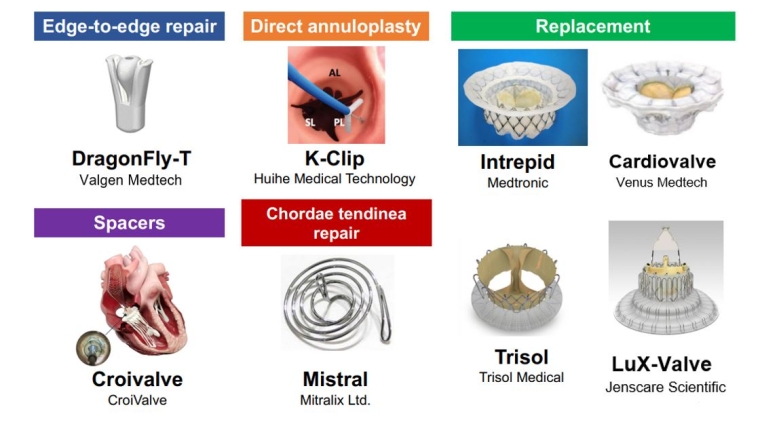
Figure 3. Proposal for device selection according to aetiology.
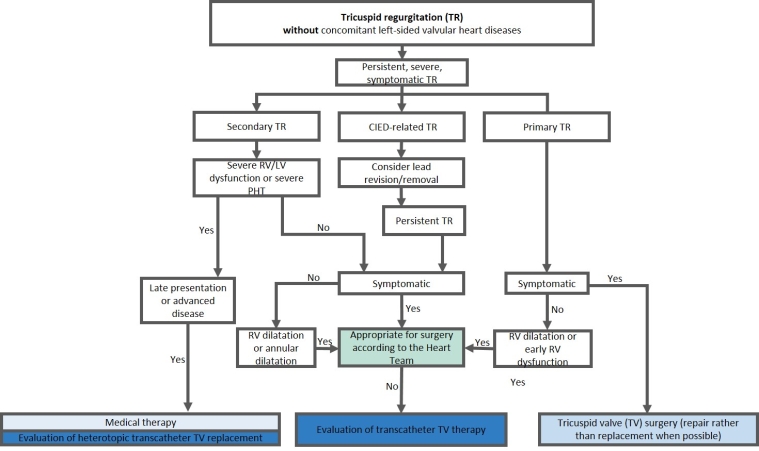
LV: left ventricular; RV: right ventricular
Transcatheter edge-to-edge repair
Tricuspid transcatheter edge-to-edge repair (T-TEER) techniques aim to increase leaflet coaptation by grasping tricuspid valve leaflets. T-TEER devices have been developed based on the initial experience of applying mitral TEER to the tricuspid valve [5,6] (Table 1). The latest generation of TriClip (TriClip G4 system; Abbott) has multiple clip sizes and allows independent leaflet grasping in order to optimise leaflet insertion. This has improved procedural results as shown in the latest data from the bRIGHT post approval study (TR reduction to ≤moderate at 30 days in 77% of the patients) [7].
Similarly, the PASCAL system (Edwards Lifesciences) has shown encouraging results in early feasibility studies in the EU and USA [8,9]. The PASCAL system consists of a central spacer that acts as a filler in the regurgitant orifice of the TV, while its nitinol construction may minimise the stress on fragile tricuspid leaflets.
Owing to its broad availability and high safety, T-TEER is the most widely used transcatheter technique for TR in the current clinical environment. The TRILUMINATE Pivotal trial – the first randomised control trial comparing T-TEER and medical therapy alone – revealed that T-TEER with the TriClip system improved quality of life in patients with TR [10]. On the other hand, clinical events, such as mortality or hospitalisation due to heart failure, were comparable between the T-TEER and medical therapy groups. Several other ongoing randomised control trials, including the CLASP II TR trial (the PASCAL system vs medical therapy), will provide further insights into the benefits of T-TEER during the year 2024 (Table 2).
Table 1. Key trials of CE-marked transcatheter devices for TR.
| Type of intervention | Study name | Design | Devices | Number of patients | TR reduction at 30 days | Post-procedural mortality | Clinical outcomes |
|---|---|---|---|---|---|---|---|
| Edge-to-edge repair |
|||||||
| TRILUMINATE Feasibility Study (NCT03227757) | Prospective, single-arm, observational study | TriClip NT | 85 |
At least 1 grade reduction: 86%, TR reduction to ≤2+: 56% |
6-month mortality: 5% |
Improvement in NYHA class, KCCQ score, and 6MWD 40% reduction of hospitalisation |
|
| CLASP TR Early Feasibility Study (NCT03745313) | Prospective, single-arm, observational study | PASCAL | 34 |
At least 1 grade reduction: 85% TR reduction to ≤2+: 52% |
30-day mortality: 0% |
Improvement in NYHA class, KCCQ score, and 6MWT at 30 days |
|
| bRIGHT Post-Approval Study (NCT04483089) | Prospective, post-market survey | TriClip G4 | 511 |
TR reduction to ≤2+: 77% |
30-day mortality: 1.0% |
Improvement in NYHA class and KCCQ score |
|
| TRILUMINATE Pivotal Study (NCT03904147) | Prospective randomized control trial (control arm: medical therapy alone) | TriClip G3 or G4 |
350 (175 for intervention) |
TR reduction to ≤2+: 87% |
30-day mortality: 0.6% |
Improvement in KCCQ score compared to medical therapy alone at 1 year |
|
| Direct annuloplasty |
|
|
|
||||
| TRI-REPAIR Study (NCT02981953) | Prospective, single-arm, observational study | Cardioband |
30 |
TR reduction to ≤2+: 76% |
30-day mortality: 6.7% |
Improvement in NYHA class, KCCQ score, and 6MWT at 6 months |
|
| Cardioband EFS (NCT03382457) | Prospective, single-arm, observational study | Cardioband |
37 |
TR reduction to ≤2+: 45.4% |
30-day mortality: 0% |
Improvement in NYHA class and KCCQ score at 1 year |
|
| TriBAND Study (NCT03779490) | Prospective, post-market survey | Cardioband |
61 |
TR reduction to ≤2+: 69% |
30-day mortality: 1.6% |
Improvement in NYHA class and KCCQ score at 30 days |
|
| Caval valve implantation |
|
|
|
||||
|
TRICUS EURO study (NCT04141137) |
Prospective, single-arm, observational study | TricValve |
35 |
– |
30-day mortality: 5.7% |
Improvement in NYHA class and KCCQ at 6 months |
|
| Replacement |
|
|
|
|
|||
|
TRISCEND Study (NCT04482062) |
Prospective, single-arm, observational study | EVOQUE system |
56 |
TR reduction to ≤1+: 98.1% |
30-day mortality: 3.6% |
Improvement in NYHA class, KCCQ score, and 6 MWT at 30 days |
|
|
TRISCEND II Pivotal trial (NCT04482062) |
Prospective randomized control trial (control arm: medical therapy alone) | EVOQUE system |
First 150 (96 for intervention) |
TR reduction to ≤1+ at 6 months: 93.8% |
30-day cardiovascular mortality: 3.2% |
Improvement in NYHA class, KCCQ score, and 6 MWT at 6 months |
KCCQ: Kansas City Cardiomyopathy Questionnaire; NYHA: New York Heart Association; TR: tricuspid regurgitation; 6 MWT: 6-minute walk test
Table 2. Upcoming landmark studies of transcatheter tricuspid valve interventions.
| Study design | Type of intervention | Study name | Countries | Registration | Study arm | Enrollment | Primary endpoint |
|---|---|---|---|---|---|---|---|
| Randomised control trials |
|||||||
| T-TEER | CLASP II TR | US | NCT04097145 |
PASCAL + OMT vs. OMT (1:1) |
870 | Composite endpoint of mortality, RVAD implantation or heart transplant, TV intervention, HF hospitalisation, and QoL improvement at 24 months | |
| T-TEER | TRI-FR | France | NCT04646811 |
T-TEER + OMT vs. OMT (1:1) |
300 | Composite endpoint of NYHA class, patient global assessment, and major cardiovascular events at 12 months | |
| Any devices | TRICI-HF | Germany | NCT04634266 |
Any CE-marked devices + OMT vs. OMT (2:1) |
360 | Composite endpoint of mortality and HF hospitalisation at 12 months | |
| TTVR | TRISCEND II Pivotal trial | US and EU | NCT04482062 |
EVOQUE + OMT vs. OMT (2:1) |
1070 | Composite endpoint of mortality RVAD implantation or heart transplant, TV intervention, HF hospitalisation, NYHA class, and 6MWD | |
| Feasibility studies |
|
||||||
| TTVR | TARGET | Germany | NCT05486832 |
Single-arm: Cardiovalve |
100 | Freedom from device or procedure-related adverse events within 30 days | |
| TTVR | TTVR Early Feasibility Study | US | NCT04433065 |
Single-arm: Intrepid |
15 | Rate of implant or delivery related serious adverse events | |
| TTVR | Study to Evaluate the Safety and Performance of LuX-Valve Plus System for TR | France | NCT05436028 |
Single-arm: Lux-Valve Plus |
135 | A composite endpoint of Major Adverse Event (MAE) at 30 days postprocedure | |
| TTVR | Trisol EFS Study | US | NCT04905017 |
Single-arm: Trisol |
15 | Rate of device-related serious adverse events | |
| Spacer | TANDEM I | Poland | NCT05296148 |
Single-arm: CroiValve |
10 | Freedom from device or procedure related serious adverse events | |
| T-TEER | Feasibility Study of the DragonFly-T System for Severe TR | China | NCT05671640 |
Single-arm: DragonFly-T |
10 | A composite of all-cause death or recurrent heart failure (HF) hospitalisations within 12 months | |
| Indirect annuloplasty | TriStar | China | NCT05173233 |
Single-arm: K-Clip |
67 | All-cause mortality and change of TR grade at 1 year | |
| Leaflet approximation | TRIBUTE | Israel | NCT05767645 |
Single-arm: Mistral device |
75 | Incidence of major device-related adverse events at 6 months |
CE: European conformity; HF: heart failure; KCCQ: Kansas City Cardiomyopathy Questionnaire; NYHA: New York Heart Association; OMT: optimal medical therapy; QoL: quality of life; RVAD: right ventricle assist device; TR: tricuspid regurgitation; TV: tricuspid valve; 6 MWT: 6-minute walk test
Direct annuloplasty
Tricuspid annulus dilation reduces leaflet coaptation and represents the main mechanism of TR progression. Direct annuloplasty devices mimic surgical tricuspid annuloplasty and aim to reduce tricuspid annular size. The Cardioband system (Edwards Lifesciences) is the first device to receive a CE (Euorpean conformity) mark as a direct annuloplasty device for the tricuspid valve position. The implantation is performed by means of multiple screw anchors and can be cinched to improve leaflet coaptation through downsizing of the tricuspid annulus. Patients with TR primarily due to tricuspid annulus dilation may be good candidates for direct annuloplasty devices. Furthermore, the direct annuloplasty allows a combined procedure with T-TEER, which may be a promising treatment option for patients with progressive TR. Early feasibility studies in the EU and USA revealed the safety and effectiveness of TR reduction by the Cardioband system (Table 1) [11,12]. A European post-market follow-up study (the TriBAND study) showed the effectiveness of the Cardioband system in real-world practice [13]. In addition, a novel direct annuloplasty technology, K-Clip (Huihe Medical Technology), which reduces the tricuspid annulus circumference by directly grasping the tricuspid annulus by a clip, is prepared for clinical trials [14].
Transcatheter tricuspid valve replacement
Despite the multiple options of repair devices, there remain concerns about significant residual TR in a non-negligible number of patients. Transcatheter tricuspid valve replacement (TTVR) may be a key treatment option to tackle this unmet need. Currently, the EVOQUE tricuspid valve replacement system (Edwards Lifesciences) is the first CE-certified TTVR system consisting of a self-expanding bioprosthetic valve that can be delivered percutaneously via the transfemoral transvenous approach. The prospective TRISCEND study reported feasibility, safety, and effectiveness of the EVOQUE system (Table 1) [15]. The ensuing TRISCEND II Pivotal Trial, a randomised control trial comparing clinical outcomes between the EVOQUE system and conservative treatment of 150 patients, demonstrated that TTVR with the EVOQUE system improved quality of life and functional capacity at six months, compared to medical therapy alone (Table 2). Notably, the EVOQUE system achieved TR reduction to ≤mild in 93.8% of the patients. This ability to eliminate TR is one of the major advantages, which may lead to better clinical outcomes. In addition, several other TTVR systems such as Cardiovalve (Venus Medtech), Intrepid (Medtronic), LuX-Valve (Jenscare Scientific) and Trisol (Trisol Medical) are currently under clinical investigation (Table 2), and promising results from first-in-human series have shown the feasibility, safety, and efficacy of these TTVR devices.
Caval valve implantation
Heterotopic tricuspid valve replacements, meaning stented valve implantation in the inferior and superior vena cava (bicaval valve implantation), are alternative treatment options in cases of far advanced TR with subsequent right ventricular remodelling and annular dilatation. Caval valve implantation can reduce backflow into the vena cava and may improve symptoms related to the venous congestion. The TricValve system (Products + Features) is a CE (European conformity)-marked device for caval valve implantation, consisting of two self-expanding valves designed for the superior and inferior vena cava. An early feasibility study (the TRICUS EURO) (Table 1) showed improvement in heart failure symptoms and quality of life after TricValve implantation [16]. However, heterotopic tricuspid valve replacement remains a bailout option for patients with anatomy ineligible for other transcatheter devices or inadequate echocardiographic imaging quality.
Postinterventional management after transcatheter tricuspid valve intervention
Transcatheter tricuspid valve repair techniques, such as T-TEER and direct annuloplasty, require life-long antiplatelet therapy after the procedures, usually with aspirin – as long as there is no other indication for oral anticoagulation. The vast majority of patients who undergo tricuspid valve interventions suffer from concomitant atrial fibrillation, and, therefore, oral anticoagulation can be continued without the need for adaptation of the treatment regimen. For patients undergoing TTVR, life-long oral anticoagulation is recommended to prevent prosthetic valve thrombosis. However, the most appropriate regimen is not yet established. The early studies of TTVR used vitamin K antagonists as the postprocedural anticoagulant treatment, while direct oral anticoagulants are recommended in the TRISCEND II trial. Since patients with TR are generally considered to be at high bleeding risk due to multiple comorbidities, further investigations are certainly needed.
During postprocedural follow-up, special attention should be paid to potential changes in volume status. Owing to a decreased venous backflow and an elevated right ventricular (RV) forward stroke volume, the venous congestion may rapidly improve, wherein reduction of diuretics might be necessary. Moreover, regular echocardiographic follow-ups should be performed to monitor right heart function and pulmonary pressures as well as dynamics of left-sided valvular diseases, which in turn might be influenced by an elevated RV forward stroke volume.
Impact on practice statement
Tricuspid regurgitation is a common valvular disease leading to a significant reduction of functional capacity and is associated with excess morbidity and mortality – if left untreated. Multiple surgical and transcatheter treatment options are currently available for TR treatment. Data regarding safety and symptomatic improvements of transcatheter treatment are encouraging. Currently ongoing randomised control trials will show the impact of these procedures on life expectancy.






 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.