Take-home messages
- TR is a common valvular disease and has an important impact on functional capacity and clinical outcomes.
- Early referral for the evaluation of a TR intervention (transcatheter or surgery) is essential to improve symptoms and quality of life.
- Detailed anatomical assessment by multimodality imaging of the tricuspid valve is essential.
- A comprehensive approach to evaluate patients with TR regarding clinical assessment and imaging is needed.
Introduction
Tricuspid regurgitation (TR) is a common finding during routine echocardiography [1]. Mild TR is detected in 75% of adults, while relevant (≥moderate) TR is estimated to affect 4% of the population over 75 years of age, which is comparable to the prevalence of aortic stenosis and mitral regurgitation [1-3]. For yet unknown reasons and in contrast to other valvular diseases, women are up to four times more frequently affected than men and the female sex is an independent predictor of the severity and progression of the disease [4-6]. This may be due to a higher prevalence of atrial fibrillation and heart failure with preserved ejection fraction (HFpEF) [7]. Besides age, sex and atrial fibrillation, pre- and postcapillary pulmonary hypertension are predictors of disease severity and progress [8].
TR manifests with impaired functional capacity and dyspnoea on exertion, as well as peripheral oedema, ascites, gastrointestinal symptoms and right heart failure, and in the advanced stages, consecutive renal and liver dysfunction [9]. The first therapeutic measure consists of the initiation of diuretic treatment.
Because of the longstanding and misleading belief that the treatment of left-sided heart disease improves TR in the majority of patients and that TR does not contribute to clinical symptoms and long-term outcomes, research on this topic has been scarce in the past [9,10]. In recent years, evidence has accumulated that significant TR is associated with an increased mortality of 40-70% after 1-4 years, even when adjusted for comorbidities [11-14]. These mortality rates are comparable to aortic stenosis [15] and results have been replicated in different study cohorts, applying not only to patients with isolated TR but also to patients with heart failure with reduced ejection fraction (HFrEF) [16].
Despite increasing evidence, TR remains a rarely assessed and treated valve disease and patients are often referred too late, when irreversible right heart failure is present and subsequent treatment is associated with increased operative risk.
Anatomical and echocardiographic considerations
The tricuspid valve (TV) is the largest and most anteriorly located heart valve separating the right atrium from the right ventricle with a complex and variable anatomy. Imaging is key in diagnosis and procedural planning. Only 54% of patients present the typical three-leaflet anatomy and around 1/3 have a four-leaflet anatomy. Separation of the posterior leaflet is the most common variation, which increases the complexity of transcatheter treatment [17]. Furthermore, the commonly used echocardiographic grading for valvular regurgitation “mild-moderate-severe” does not adequately take into account the late stages of the disease and TR reduction after transcatheter treatment. In order to better address TR severity a new grading system has been proposed, adding two additional categories “massive” and “torrential” [18].
Figure 1. Echocardiographic evaluation and grading of tricuspid regurgitation (TR).
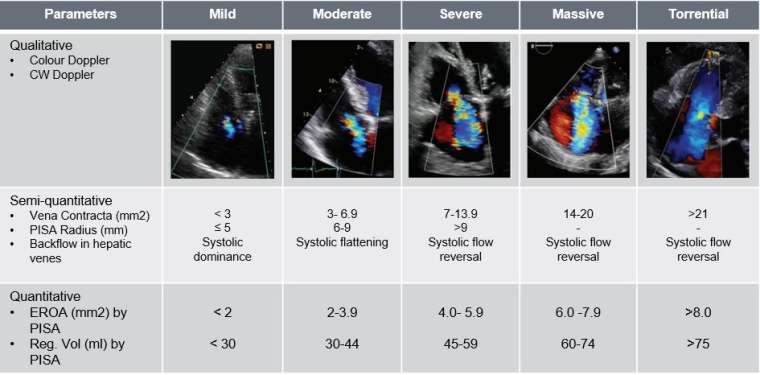
EROA: effective regurgitant orifice area; PISA: proximal isovelocity surface area
Classification and aetiology of TR
TR is classified according to its underlying pathomechanism into primary and secondary TR (STR). Primary TR (PTR) is rare, presenting in fewer than 10% of cases and is associated with abnormal leaflet morphology. Usually, PTR is observed in congenital or rheumatic heart disease or can be the consequence of infective endocarditis [19]. Infrequently repeated myocardial biopsies after (orthotropic) heart transplant can lead to PTR [20].
STR is the more prevalent pathomechanism and is due to changes in the atrial and/or ventricular geometry, leading to tethering of the leaflets or dilatation of the annulus [21] and is usually caused by left heart disease or pulmonary hypertension [22]. Atrial STR is newly recognised as a separate aetiology, due to long-standing atrial fibrillation (and age) with atrial remodelling [21]. With more cardiac electronic devices being implanted, cardiac implantable electronic devices (CIED)-related TR has increased and represent a distinct entity necessitating interdisciplinary approach and management [23,24].
Figure 2. Aetiological classification of TR.
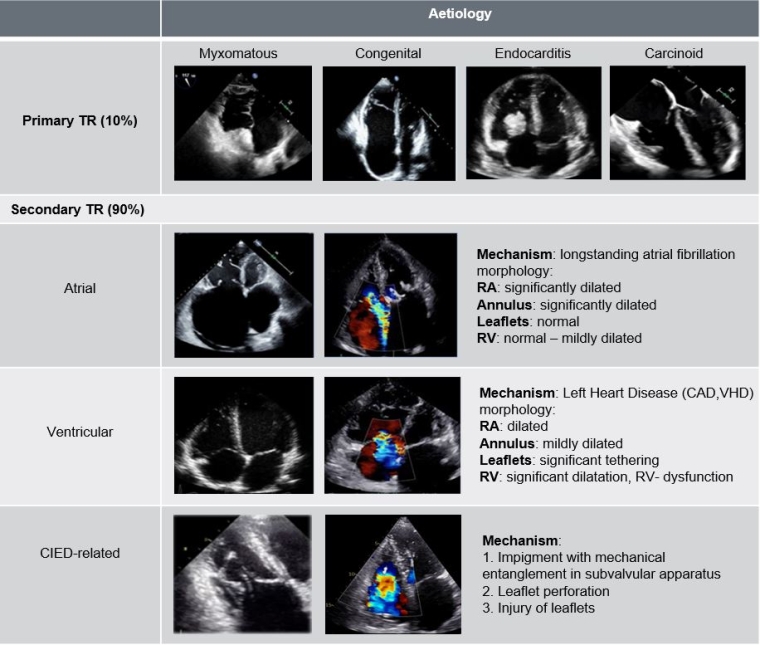
CAD: coronary artery disease; CIED: cardiac implantable electronic devices; RA: right atrium; RV: right ventricle; TR: tricuspid regurgitation; VHD: valvular heart disease
Risk stratification/ Guideline recommendations
With an ageing population and more minimally invasive treatment options available, the need to predict mortality, in order to adequately select patients who will benefit from an intervention or who are eligible for surgery, is crucial. Since neither the Society of Thoracic Surgeons (STS) score nor the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II were designed to predict the risk of isolated TV interventions, a new dedicated score has been proposed for isolated tricuspid surgery [25,26] based on clinical predictors (Table 1) [25].
Figure 3. TRI-Score: prediction of in- hospital mortality after isolated TV-surgery.
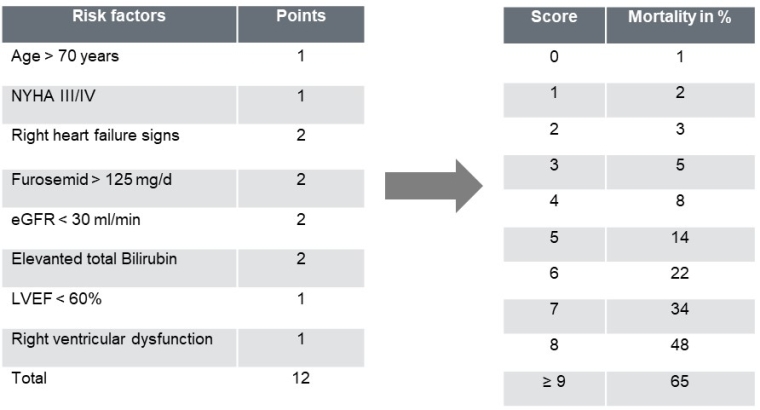
eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association
There is no consensus about the best strategy and patient population for isolated TV interventions. Current European guidelines recommend TV surgery for severe primary/secondary TR when undergoing left heart surgery (Class Ia indication). Isolated TV surgery is indicated for severe, symptomatic TR (Class Ia indication). Patients with severe, asymptomatic (or oligosymptomatic) TR without RV dysfunction or RV dilatation also have a Class IIa recommendation [31].
Conclusion
Isolated tricuspid valve surgery is still considered high risk with in-hospital mortality rates ~10% and presenting high mortality during follow-up (major adverse events occur in about ~30% of patients with advanced disease) [27-30]. These results correlate primarily with the severity of TR rather than its aetiology, leading to the conclusion that early referral is essential in order to improve outcomes.
Figure 4. Comprehensive approach to evaluate patients with TR with multimodal cardiac imaging and clinical assessment.
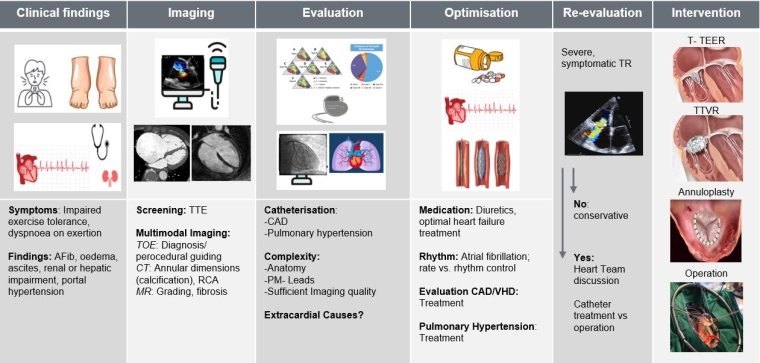
CAD: coronary artery disease; CT: computed tomography; MR: mitral regurgitation; RCA: right coronary artery; TOE: transoesophageal echocardiography; TR: tricuspid regurgitation; TTE: transthoracic echocardiography; T-TEER: tricuspid transcatheter edge-to-edge repair; TTVR: transcatheter tricuspid valve replacement; VHD: valvular heart disease
Impact on Practice
Severe, symptomatic tricuspid regurgitation has an important impact on clinical outcomes and needs to be addressed early in order to improve symptoms and quality of life, therefore early referral to a specialised centre is of major importance.






 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.