Key words
Acute coronary syndrome; adherence; beta-blocker; clinical management; survival
Abbreviation list
ACS: acute coronary syndrome
CABG: coronary artery bypass grafting
CNIC: Spanish National Centre for Cardiovascular Research
DAPT: dual antiplatelet therapy
DOACs: direct oral anticoagulants
HbA1C: glycated haemoglobin
HDL-C: high-density lipoprotein-cholesterol
LDL-C: low-density lipoprotein-cholesterol
LVEF: left ventricular ejection fraction
PCSK9: proprotein convertase subtilisin/kexin type 9
QOL: quality of life
WHO: World Health Organization
Take-home messages
- An extended course of dual antithrombotic therapy should be considered in individuals with high ischemic risk without high bleeding risk or major bleeding complications.
- After the first acute coronary syndrome event, the goal is to lower LDL-C to <1.4 mmol/L (<55 mg/dL) and to reduce LDL-C levels ≥50% from baseline.
- Beta-blockers are recommended in patients with LVEF ≤40% after an acute coronary syndrome, regardless of heart failure symptoms.
- A polypill strategy should be considered to improve adherence and cardiovascular outcomes after an acute coronary syndrome.
Patient-oriented message
After an acute coronary syndrome, secondary prevention should be offered to all patients and start as soon as possible after the index event in order to avoid complications such as the occurrence of another acute coronary syndrome. Long-term management includes psycho-social counselling, pharmacological treatment optimisation and adherence as well as cardiac rehabilitation and has been proven to both increase the quality of life and decrease morbidity and mortality.
It is of paramount importance to include patients in the decision-making. Educating and informing the patient properly and employing appropriate material should be integrated into the standard patient care pathway.
Introduction
Secondary prevention after an acute coronary syndrome (ACS) is key to improving quality of life (QOL) as well as to diminishing morbidity and mortality rates. This should consist of a multimodal approach, including cardioprotective medications, lifestyle management and cardiac rehabilitation referral, all of which should start as soon as possible after the index event. In order for these changes to be implemented by the patient, an outpatient clinical appointment should be arranged, so as to review the patient´s objectives and preferences, as well as to review the management of the different comorbidities they may suffer from.
According to the 2023 ESC Guidelines for the management of acute coronary syndromes [1], treatment goals con be divided into 3 subgroups:
- Support of healthy lifestyle pathways, such as regular exercise, smoking cessation and psychosocial management, among others.
- Continuation of optimal medical therapy, including annual influenza vaccination, promoting medication adherence, lipid-lowering therapy and antithrombotic therapy.
- Achieve the risk factor treatment targets including, but not limited to blood pressure, low-density lipoprotein-cholesterol (LDL-C) levels as well as glycated haemoglobin (Hba1C) levels.
Even though the approach to successful long-term management is multimodal, this article will focus mainly on four areas: antithrombotic therapy, lipid-lowering therapy, beta-blocker therapy and the role of pharmacological adherence, after an ACS.
Importance of long-term risk assessment before discharge
Prior to discharge, it is of paramount importance for every patient to undergo a thorough long-term risk assessment which should include left ventricular ejection fraction (LVEF), residual ischaemia, complexity of coronary artery disease as well as completeness of revascularisation, in-hospital complications, and analytic risk markers such as LDL-C, high-density lipoprotein cholesterol (HDL-C), fasting triglycerides, plasma glucose and renal function status. It is important to measure LDL-C upon the patient´s admission to the hospital, as these levels tend to decrease in the subsequent days after an ACS.
It is relevant to note that if successful reperfusion is not performed, the risk of early complications and death increases significantly. In this particular subset of patients, residual ischaemia should be assessed and, when indicated, myocardial viability as well.
Long-term management of antithrombotic therapy
Typically, the standard approach for the majority of patients is dual antiplatelet therapy (DAPT) for at least one year following an ACS episode. DAPT combines a strong P2Y12 receptor blocker with aspirin. Nevertheless, certain exceptions, such as urgent surgery, concomitant anticoagulation therapy and patients at high bleeding risk, can prompt different treatment strategies.
In patients requiring anticoagulation for other reasons (e.g. atrial fibrillation, mechanical prosthetic valves…), it is recommended to use triple antithrombotic therapy up to one week after the ACS, and DAPT (with anticoagulation and single antiplatelet therapy) for the first 12 months (Class I). Anticoagulation monotherapy (Class IIb) may also be considered from 6 to 12 months. Beyond 12 months, there is a Class I recommendation for oral anticoagulation therapy [1]. Direct oral anticoagulants (DOACs) are preferred over vitamin K antagonists if the underlying condition permits the use of these agents.
When coronary artery bypass grafting (CABG) is indicated in patients with ACS with an established indication for oral anticoagulation, triple antithrombotic therapy should be avoided and anticoagulation, in combination with single antiplatelet therapy should be re-started as soon as possible [1].
After the first 12 months, aspirin monotherapy, at a maintenance dose of 75-100 mg o.d., should be used for all patients if no contraindications are present, with a Class I recommendation, Level of Evidence B.
According to the results of the Dual Antiplatelet Therapy (DAPT) [2] and PrEvention with TRicaGrelor of SecondAry Thrombotic Events in High-RiSk Patients with Prior AcUte Coronary Syndrome-Thrombolysis in Myocardial Infarction Study Group (PEGASUS-TIMI) 54 [3] trials; an extended course of DAPT beyond the first 12 months after an ACS, can be an option in certain types of individuals:
- patients at high ischaemic risk and without an additional risk of life-threatening or major bleeding events (Class IIa recommendation, Level of Evidence A).
- patients with moderately increased ischaemic risk who have undergone previous DAPT treatment without a bleeding complication (Class IIb recommendation, Level of Evidence A).
Regarding the second antithrombotic agent, one can add either ticagrelor 60 mg b.i.d., which has demonstrated a lower bleeding risk compared to the 90 mg b.i.d. [4,5]; or a very low dose of rivaroxaban (2.5 mg b.i.d.) [6].
Lastly, antithrombotic monotherapy with a P2Y12 inhibitor may be considered instead of aspirin monotherapy for long-term treatment [7]. The decision tree is summarised in Figure 1.
Figure 1. Schematic representation of the long-term antithrombotic therapy management. A second antithrombotic drug in addition to aspirin can be added for extended secondary prevention in patients without high bleeding risk.
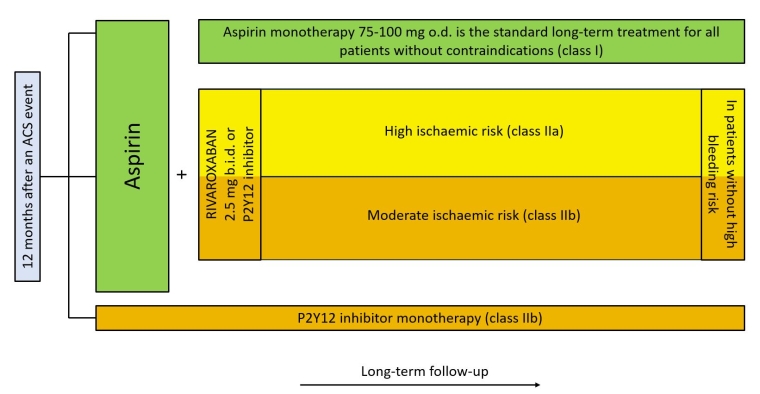
ACS: acute coronary syndrome
Long-term management of lipid-lowering therapy
Dyslipidaemia is best treated with a combined approach including pharmacological and lifestyle interventions. In secondary prevention after an ACS, the goal is to lower LDL-C to <1.4 mmol/L (<55 mg/dL) and to reduce LDL-C levels ≥50% from baseline [8-10].
After the in-hospital treatment, described in the 2023 ESC Guidelines for the management of acute coronary syndromes [1], lipid levels should be re-evaluated at 4-6 weeks, to assess whether treatment goals have been achieved and to evaluate the clinical and analytical tolerance of the drugs and their secondary effects.
If the maximum-tolerated dose of a high-intensity statin alone (e.g. atorvastatin or rosuvastatin) does not achieve LDL-C goals after 4-6 weeks following an ACS event, adding ezetimibe is recommended, with a Class I recommendation. A proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor should be started in patients who, despite the maximum-tolerated dose of statin and ezetimibe therapy, do not reach their LDL-C goal.
Additionally, if triglyceride levels in patients after an ACS, despite statin treatment, are still elevated: 1.5-1.6 mmol/L (135-499 mg/dL), icosapent ethyl, at a dose of 2 g b.i.d. can be initiated in combination with statin, to reduce the residual risk [8,11].
Finally, in recurrent events during follow-up, within 2 years after the index episode, a lower LDL-C goal of <1 mmol/L (<40 mg/dL) seems to offer additional benefit [8-10].
Long-term management of beta-blockers on discharge
The clinical benefit of beta-blockers in patients with LVEF <40% has been proven in contemporary trials [12,13], regardless of heart failure symptoms, with a Class I recommendation and Level of Evidence A.
However, the role of beta-blockers in patients with LVEF >40% after an ACS event without additional complications is not well established, as the vast majority of randomised clinical trials assessing the benefit of beta-blockers after an ACS were performed in the pre-reperfusion era. The results of the REDUCE-SWEDEHEART [14] have recently been published and suggest that long-term beta-blocker therapy might not lead to a lower risk of the composite endpoint of death from any cause or new myocardial infarction, compared to no beta-blocker use. However, this trial was terminated earlier than originally planned and as a result it is an overtly underpowered trial.
There are two larger, adequately powered trials that have finished enrolment and will report their primary results in 2025: REBOOT-CNIC [15], and the BETAMI-DANBLOCK [16]. Figure 2 summarises the relevant trials. Until these two trials are reported, maintenance beta-blocker therapy should be considered for patients after an ACS without reduced ejection fraction (and it is always recommended if the LVEF is below 40%).
Figure 2. Schematic representation of the three clinical trials in Europe in patients after an acute myocardial infarction without reduced LVEF. Note that the REDUCE-SWEDEHEART study includes patients with LVEF >50%, whereas REBOOT-CNIC, and the BETAMI-DANBLOCK enrol patients with LVEF >40%.
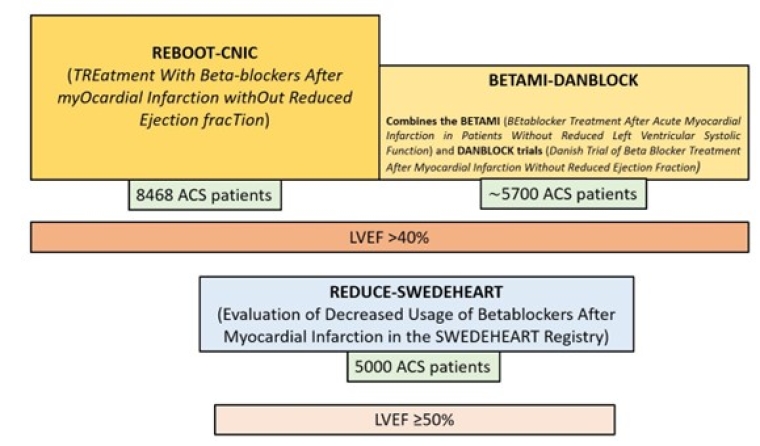
ACS: acute coronary syndrome; LVEF: left ventricular ejection fraction
The role of optimal medication adherence after an ACS
Adherence to medication is key in preventing recurrent cardiovascular events after an ACS and is only achieved in about two-thirds of the patients in secondary prevention. Contributing factors to this suboptimal adherence include polypharmacy and drug regimen complexity, among others [1].
Polypills, including guideline-recommended medical therapy for secondary prevention may improve therapeutic targets and improve adherence for secondary prevention post-ACS [17,18]. The Secondary Prevention of Cardiovascular Disease in the Elderly (SECURE) randomised clinical trial is the first and only one to assess the impact of a polypill-based strategy (containing atorvastatin, aspirin and ramipril) versus usual care on hard outcomes in patients with ACS. The polypill approach was associated with a significant 33% reduction in cardiovascular mortality [19]. Thus, a polypill strategy should be considered to improve adherence and cardiovascular outcomes after an ACS [1] (Class of recommendation IIa, Level of Evidence B).
The World Health Organization (WHO) has echoed this clinically relevant finding and has included the cardiovascular polypill, developed by the Spanish National Centre for Cardiovascular Research (CNIC) and the pharmaceutical company Ferrer, in its 23rd List of Essential Medicines (2023) [20].
Conclusions/Impact on practice statement
Risk stratification prior to discharge in patients after an ACS is helpful in determining whether an antithrombotic therapy should be prolonged or not, and which strategy to use, according to the risk-benefit balance. Moreover, LDL-C should be quickly measured upon arrival and periodically assessed during follow-up visits, aiming for LDL-C <55 mg/dL after the first ACS event (Class I, Level of Evidence A). Beta-blockers are recommended in patients with LVEF ≤40% after an ACS event, regardless of heart failure symptoms (Class I, Level of Evidence A). Lastly, a polypill strategy should be considered to improve adherence and cardiovascular outcomes after an ACS (Class IIa, Level of Evidence B).



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.