Take-home messages
1. Hot Line 1. The safety of βB interruption in MI patients with preserved LVEF is not demonstrated. No difference in post-operative MACE between continuing/discontinuing RAS inhibitors before surgery.
Hot Line 1 reported on by Dr Dimitri Richter, FESC (Athens, Greece)
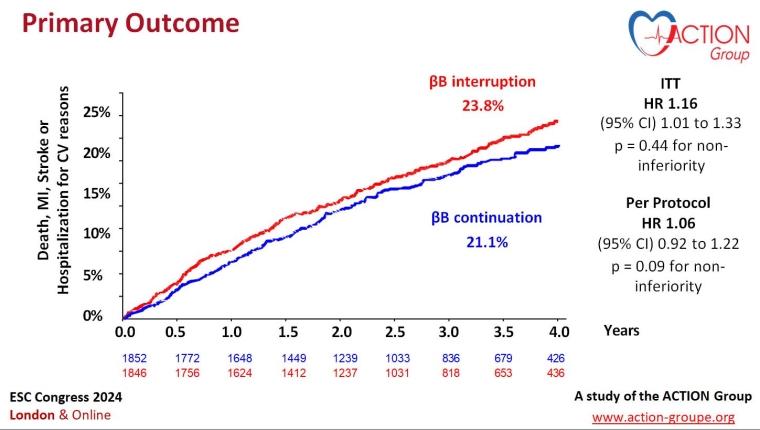
ABYSS: Beta blocker interruption in post-myocardial infarction patients
In this French trial, 3,698 patients with an ejection fraction >40%, receiving long-term beta blocker (βB) treatment and with no history of cardiovascular events in the six months prior to enrolment, were randomised to either interruption (n=1,846) or continuation (n=1,852) of beta blocker therapy.
The primary endpoint was a composite of death, non-fatal myocardial infarction (MI), non-fatal stroke, or hospitalisation for cardiovascular reasons at a minimum of one year.
A primary outcome event occurred in 432 (23.8%) patients in the interruption group compared with 384 patients (21.1%) in the continuation group (p=0.44 for non-inferiority).
ABYSS did not demonstrate the safety of βB interruption in MI patients with preserved left ventricular ejection fraction. βB interruption did not improve patients’ quality of life and increased their blood pressure and heart rate.
In the related NEJM editorial, Tomas Jernberg wrote that it is prudent to wait for the results of additional ongoing trials that, added to the results of ABYSS and REDUCE-AMI, will most likely provide firm evidence regarding beta blocker treatment in this patient population.
Silvain J, et al. N Engl J Med. 2024. Aug 30. doi: 10.1056/NEJMoa2404204. Epub ahead of print. PMID: 39213187.
STOP-or-NOT: Impact of renin-angiotensin system inhibitors continuation versus discontinuation on postoperative complications: a multicentre randomised, controlled trial
This trial was conducted in France and randomised 2,222 patients (mean age 67 years, 65% male) to either continue renin-angiotensin system inhibitors (RASIs) until the day of major elective non-cardiac surgery or discontinue them 48 hours prior to the procedure. At baseline, 98% percent of patients were receiving treatment for hypertension, 9% had chronic kidney disease, 8% had diabetes, 4% had heart failure, 46% were treated with angiotensin-converting enzyme inhibitors (ACEIs) and 54% were treated with angiotensin receptor blockers (ARBs). In both groups, it was recommended that RASIs resume as soon as possible after surgery.
The rates of postoperative major cardiovascular events within 28 days after surgery were the same (22%) across both the discontinuation and continuation groups. The effect of discontinuation versus continuation of RASIs on the risk of postoperative complications was consistent across subgroups. Episodes of hypotension during surgery occurred in 41% of patients in the discontinuation group compared with 54% in the continuation group.
Given the lack of difference, both strategies appear acceptable, indicating that a tailored approach to RASI continuation could be used.
Legrand M, et al. JAMA. 2024 Aug 30:e2417123. doi: 10.1001/jama.2024.17123. Epub ahead of print. PMID: 39212270; PMCID: PMC11365013.
Access the session here : HOT LINE 1
Hot Line 2 reported on by Luigina Guasti, MD, PhD, FESC (Varese, Italy)
Bedtime versus morning dosing of antihypertensive medications in relation to clinical outcomes and major adverse cardiovascular events (MACE) has been a controversial issue in recent years.
Two studies and a meta-analysis were focused on the effect of antihypertensive administration time on MACE and on safety.
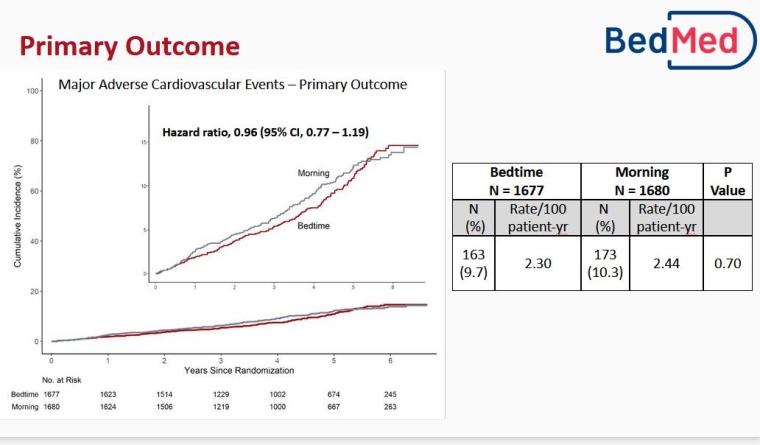
BedMed: Antihypertensive medications efficacy – just as good in the morning as at bedtime!
In the BedMed study, Scott Garrison (Edmonton, Canada) and colleagues recruited community dwelling hypertensive patients with ≥1 ongoing blood pressure (BP) medications using Canadian administrative health data.
Patients were asked to take medication when getting ready for bed whereas the control group was asked to take all once-daily BP medications upon waking in the morning. The primary endpoint was all-cause death, hospital emergency department visit for stroke, acute coronary syndrome and heart failure. A secondary endpoint concerning safety was also reported.
In all, 3,357 patients (mean age 67 years) were randomised into two groups (bedtime versus morning uptake). Adherence to allocation was 83% for bedtime versus 95% in the morning at 6 months, with the greatest off allocation for diuretics, possibly due to the discomfort associated with urination at night. After a 4.6 years median follow-up, no difference in MACE and no difference in any outcome was observed.
BedMed-Frail: Effect of antihypertensive timing on mortality and morbidity
The BedMed-Frail trial studied hypertensive patients in continuing care. The median age was 88 years and patients were on 1-2 BP medications. 829 patients were randomised in an open protocol in two groups (morning versus bedtime uptake). No substantial difference was observed in outcomes or in potential adverse effects.
A systematic review and meta-analysis
A systematic review and meta-analysis was conducted by Ricky Turgeon (Vancouver, Canada), including a total of 46,606 patients from 5 studies (BedMed, BedMed-Frail, Hygia, MAPEC and TIME), comparing evening administration of ≥1 antihypertensive treatment to morning uptake. No difference was observed in MACE or in safety outcomes according to the two different timings of medication adherence.
Access the session here : HOT LINE 2
Hot Line 3 reported on by Riccardo Asteggiano, MD (Varese & Turin, Italy)
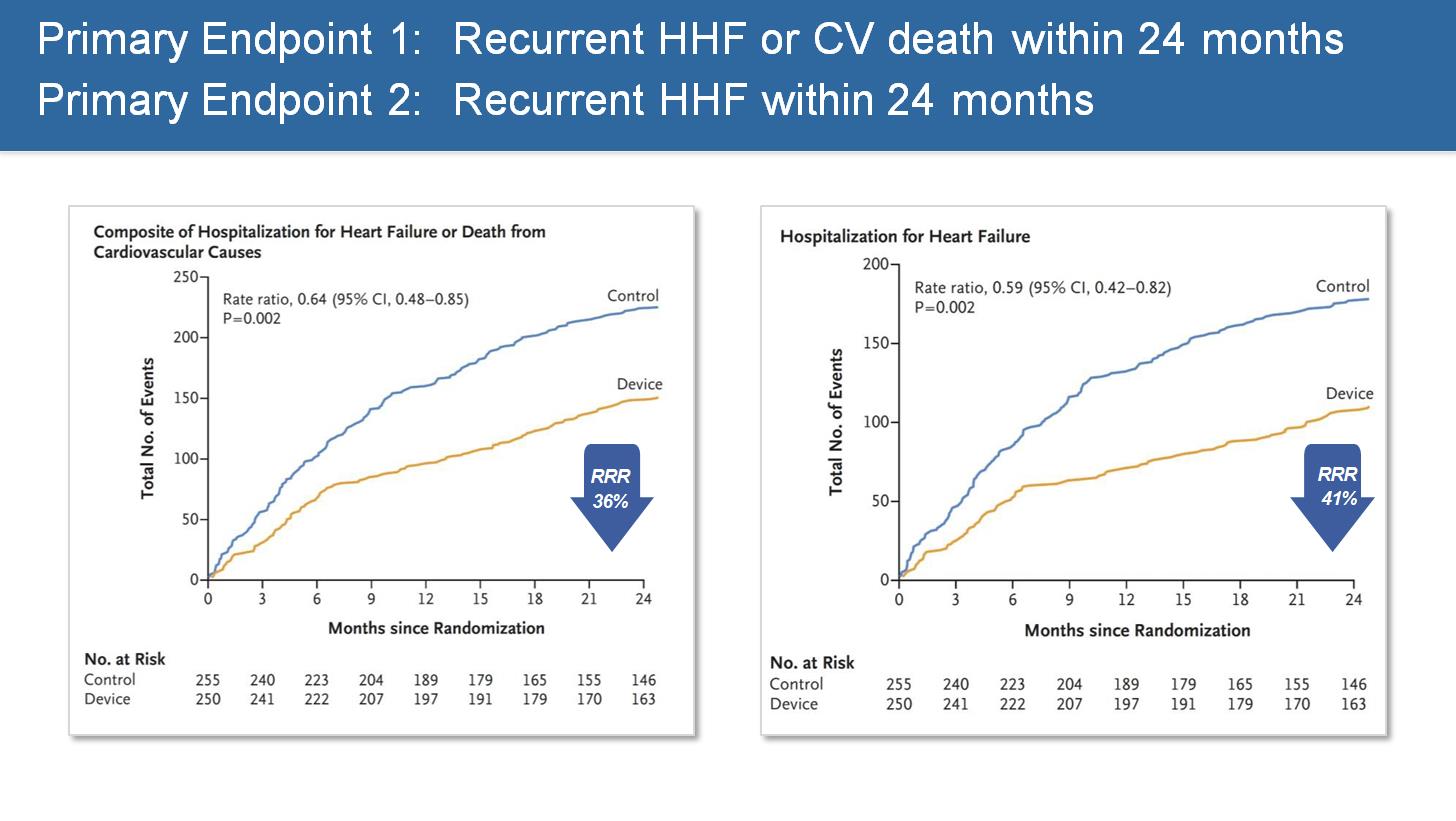
RESHAPE-HF2: Percutaneous repair of moderate-to-severe or severe functional mitral regurgitation in patients with symptomatic heart failure
Professor Stefan D. Anker (Berlin, Germany) presented the results of a randomised controlled trial on 505 patients with heart failure (HF) and moderate-to-severe functional mitral regurgitation under medical therapy. The addition of transcatheter mitral valve repair led to a lower rate of first or recurrent hospitalisation for HF or cardiovascular (CV) death and a lower rate of first or recurrent hospitalisation for HF at 24 months (37.0 events per 100 patient-years in the device group and 58.9 events per 100 patient-years in the control group (rate ratio 0.64, [95% CI: 0.48-0.85]; p=0.002).
Anker SD, et al. N Engl J Med. 2024 Aug 31. doi: 10.1056/NEJMoa2314328. Epub ahead of print. PMID: 39216092.
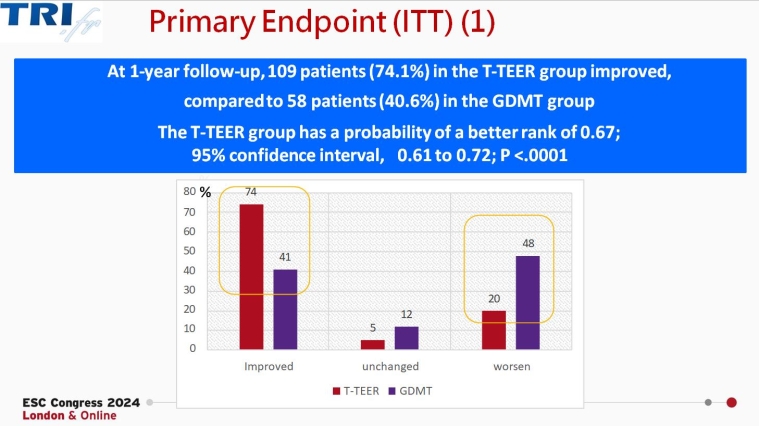
Tri.fr: Multicentric randomised evaluation of transcatheter edge-to-edge repair in the treatment of severe isolated secondary tricuspid regurgitation
The trial, presented by Erwan Donal (Rennes, France), randomised 300 patients with symptomatic, severe secondary tricuspid regurgitation despite medical management to receive either tricuspid transcatheter edge-to-edge repair (T-TEER) in addition to optimal medical treatment or to optimal medical treatment alone. The primary endpoint (Packer composite score) rate improvements were higher among those receiving T-TEER compared with those receiving medical therapy alone (74.1% vs 40.6%, respectively). In addition, lower rates of hospitalisation and death and improvements in patients’ quality of life were noted.
Access the session here : HOT LINE 3
Hot Line 5 reported on by Alexander Kharlamov, MD, FESC (Heerhugowaard, the Netherlands)
NOTION-3: PCI in patients undergoing transcatheter aortic valve implantation
The NOTION-3 trial, presented by J.T. Lønborg (Copenhagen, DK), is a multicentre, open-label, randomised superiority trial designed to test the hypothesis that routine revascularisation of coronary artery stenoses (defined by an FFR <0.80 or a coronary artery diameter stenosis >90% as determined by angiography) would improve clinical outcomes in patients with stable coronary artery disease (CAD) and severe symptomatic aortic stenosis undergoing transcatheter aortic valve implantation (TAVI).
In a previous trial (ACTIVATION), comparable rates of death and rehospitalisation between patients undergoing percutaneous coronary intervention (PCI) and those who did not undergo PCI before TAVI were noted, but the non-inferiority threshold was not achieved and PCI was associated with a higher incidence of bleeding. In the NOTION-3 trial, 227 of the 455 randomised patients were allocated to the PCI group and 228 to the conservative treatment group. After a median follow-up of 2 years, major adverse cardiac events were observed in 60 patients (26%) in the PCI group and in 81 patients (36%) in the conservative treatment group (hazard ratio 0.71, [95% CI: 0.51-0.99]; P=0.04); these results were driven by the reduction in MI and the need for urgent revascularisation. Bleeding events were reported in 64 patients (28%) in the PCI group and in 45 patients (20%) in the conservative treatment group (hazard ratio 1.51, [95% CI: 1.03-2.22]).
The authors suggest that PCI should be considered the preferred treatment for TAVI patients with CAD, though the final decision should be individualised, based on factors such as patient age, comorbidities, life expectancy, and bleeding risk.
The RHEIA trial, presented by Hélène Elthchaninoff (Rouen, France), is a prospective, randomised, controlled, multicentre study, comparing TAVI and surgical aortic valve replacement in women with severe aortic stenosis. TAVI appeared to be superior to surgery in reducing the primary endpoint (8.9% vs 15.6%; HR 0.55, [95% CI:0.34-0.88]; p=0.03), essentially due to the reduction in rehospitalisation. Additionally, less new-onset AF and shorter initial hospital stays were associated with TAVI. However, higher rates of vascular complications, pacemaker implantation and mild paravalvular leaks were noted in the TAVI group.
Although longer-term data are needed, the findings suggest that with good preprocedural planning, proper valve selection and a tailored implantation, TAVI might be the preferred treatment for women with severe symptomatic aortic stenosis.
Lønborg J et al. NOTION-3. N Engl J Med. 2024 Aug 31. doi: 10.1056/NEJMoa2401513. Epub ahead of print. PMID: 39216095.
Patterson T, et al. ACTIVATION. JACC Cardiovasc Interv. 2021 Sep 27;14(18):1965-1974. doi: 10.1016/j.jcin.2021.06.041. PMID: 34556269
Access the session here : HOTLINE 5
HotLine 6 reported on by Thomas Kümler (Herlev, Denmark)
STEEER-AF: Stroke prevention and rhythm control therapy
Dipak Kotecha (Birmingham, UK) presented the STEEER-AF trial, which was supported by the ESC, the European Heart Rhythm Association (EHRA) and the ESC Council on Stroke. This multicentre, international, cluster randomised trial included 6 European countries and investigated whether focused education directed to healthcare professionals could improve care for patients with atrial fibrillation (AF), in line with guideline recommendations. A total of 1,732 patients were enrolled. The educational intervention was in the form of a short online programme which took a median of 9.2 hours to complete. The programme included phases of reflection and planning as well as implementation. Routine clinical follow-up was done between 6- and 9-months following randomisation.
At baseline, only 61% of patients were fully adherent to stroke prevention and 21% to rhythm control guidelines. At follow-up, there was a non-significant increase in adherence to stroke prevention but a 12% absolute significant increase in adherence to rhythm control.
Guideline adherence in routinely treated AF patients is low but can be improved through the targeted education of healthcare professionals, especially when substantial gaps in implementation are present. The results have been incorporated into the 2024 Guidelines for the management of atrial fibrillation, published at the ESC Congress.
OCEANIC-AF: Asundexian versus apixaban in patients with atrial fibrillation
Manesh Patel from Duke University Medical Center (Durham, NC, USA) presented the OCEANIC-AF trial, which randomised 14,820 of a planned 18,000 AF patients with an indication for oral anticoagulation to the factor XIa inhibitor asundexian compared to apixaban. The trial was stopped in November 2023 due to the inferiority of asundexian for the prevention of stroke and systemic embolism. There was a significant decrease in ISTH major bleeding in the asundexian group. The asundexian therapy was well tolerated.
The major question in the trial was whether the dose and inhibition of factor XI activity was sufficient. However, there was no observed difference in factor XI activity between patients with and without primary efficacy events. Results of similar ongoing trials with factor XI inhibition (including LIBREXIA-AF) are expected.
Piccini JP, et al. N Engl J Med. 2024 Sep 1. doi: 10.1056/NEJMoa2407105. Epub ahead of print. PMID: 39225267.
EPIC-CAD: Edoxaban monotherapy versus dual antithrombotic therapy for atrial fibrillation and stable coronary artery disease
Presented by Gi-Byoung Nam (Seoul, South Korea), the EPIC-CAD trial was an investigator-initiated, multicentre, open-label, superiority trial including 1,038 patients with stable coronary artery disease and AF with a CHADS2DS2-VASc score >2. After 1 year of follow-up, there was a significant decrease in the primary endpoint of net adverse clinical events in the edoxaban monotherapy group. There was no significant difference in efficacy endpoints, but there was a significant decrease in the bleeding endpoint.
These findings are in accordance with the previous AFIRE trial, supporting oral anticoagulation therapy as monotherapy in patients with AF and stable coronary artery disease.
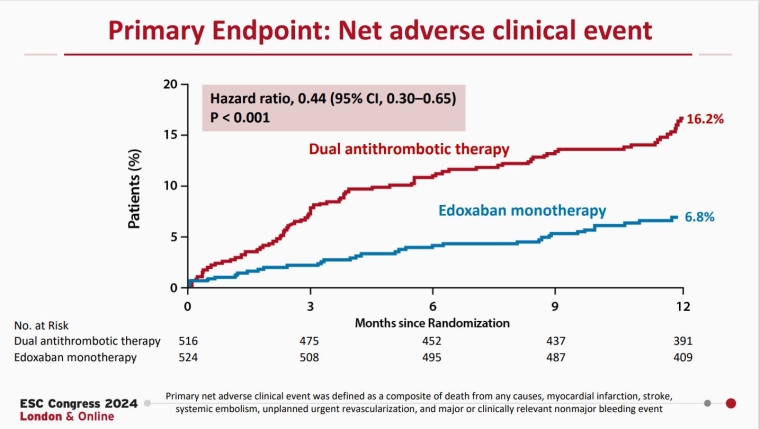
Cho MS, et al. N Engl J Med. 2024 Sep 1. doi: 10.1056/NEJMoa2407362. Epub ahead of print. PMID: 39225258.
Access the session here : HOTLINE 6
Hot Line 7 reported on by Ruxandra Christodorescu (Timişoara, Romania)
Findings from a new trial and two pooled analyses examining the use of mineralocorticoid receptor antagonists (MRAs), in particular the non-steroidal MRA finerenone, in patients with heart failure (HF) with mildly reduced or preserved EF, and across the cardio-kidney-metabolic spectrum, were presented.
FINEARTS-FR: Finerenone in heart failure with mildly reduced or preserved ejection fraction
Professor Scott Solomon (Boston, MA, USA) discussed the FINEARTS-HF trial, a double-blind study involving 6,001 patients with HF with mildly reduced or preserved ejection fraction (HFmrEF/HFpEF). Over a median follow-up of 32 months, finerenone significantly reduced the primary endpoint of total worsening HF events and CV death. Finerenone notably reduced worsening HF events compared to placebo, though it had no significant impact on CV death. There were no significant differences in all-cause mortality or composite kidney outcomes between the groups. Serious adverse events were similar, but finerenone increased the risk of hyperkalaemia while reducing the risk of hypokalaemia. This trial provides the first clear evidence of MRA benefit in HFmrEF/HFpEF patients, positioning finerenone as a potential second treatment pillar alongside SGLT2 inhibitors for this condition.
Solomon S, et al. N Engl J Med. 2024 Sep 1. doi: 10.1056/NEJMoa2407107. Epub ahead of print. PMID: 39225278
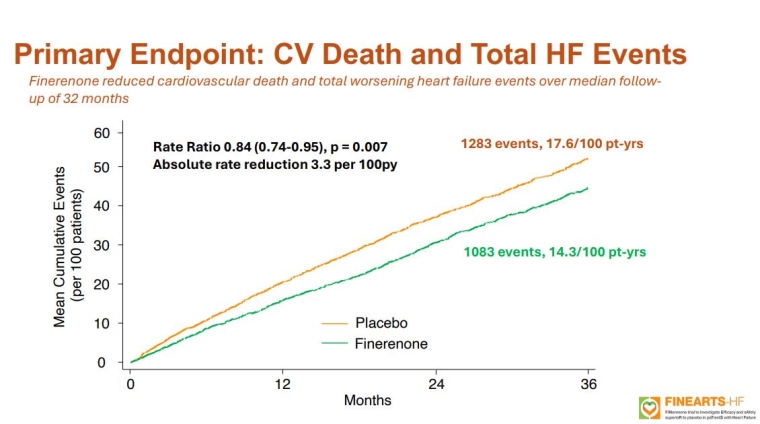
Professor Pardeep Jhund (Glasgow, UK) followed with a meta-analysis of four placebo-controlled MRA trials in HF, including all EFs, and including the previously discussed FINEARTS-HF trial. In total, 13,846 patients were analysed, and MRAs were found to reduce the risk of CV death or HF hospitalisation, with a greater benefit observed in HFrEF compared to HFmrEF/HFpEF. The analysis confirmed MRA benefits across the HF spectrum, suggesting MRA treatment should be considered for all HF patients without contraindications.
Jhund PS, et al. Lancet. 2024 Sep 21;404(10458):1119-1131. doi: 10.1016/S0140-6736(24)01733-1. Epub 2024 Sep 1. PMID: 39232490.
Lastly, Dr Muthiah Vaduganathan (Boston, MA, USA) presented the FINE-HEART analysis, which pooled data from three large finerenone trials: FIDELIO-DKD, FIGARO-DKD, and FINEARTS-HF. This participant-level analysis involved 18,991 patients. Over a median follow-up of 2.9 years, finerenone did not significantly reduce CV death, but did reduce all-cause mortality, HF hospitalisations and composite kidney outcomes. Despite a higher incidence of hyperkalaemia, finerenone demonstrated its broader disease-modifying role across the cardio-kidney-metabolic spectrum in HF high-risk patient populations.
Vaduganathan M, et al. Nat Med. 2024 Sep 1. doi: 10.1038/s41591-024-03264-4. Epub ahead of print. PMID: 39218030.
Access the session here : HOTLINE 7








 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.