Keywords
aortic stenosis; cardiac amyloidosis; heart failure with preserved ejection fraction; TAVI; transthyretin amyloidosis
Abbreviation list
AS: aortic stenosis
ATTR: transthyretin amyloidosis
CA: cardiac amyloidosis
LVH: left ventricular hypertrophy
TAVI: transcatheter aortic valve implantation
Take-home messages
- ATTR is highly prevalent in patients with severe aortic stenosis (AS) estimated at ~11%.
- Systematic screening of AS patients contributes to the identification of latent ATTR cases.
- Increased vigilance for red flags of ATTR is needed to refer patients for screening promptly.
- Early diagnosis of ATTR and initiation of TTR-targeted treatment can significantly improve patients’ prognosis and quality of life.
Introduction
Transthyretin amyloidosis (ATTR) has been increasingly recognised in recent years as a major cause of heart failure (HF). The disease remains, however, largely underdiagnosed with poor overall prognosis [1,2]. Aortic stenosis (AS) is considered the most common valvular heart disease in the elderly [3,4]. Aging demographics in the developed world have led to a significant increase in the number of patients diagnosed with AS [3], which is associated with significant morbidity and mortality [5].
Specific patient populations, including patients with severe AS and patients with HF and preserved ejection fraction (HFpEF) are more frequently affected by ATTR (Figure 1) [1,2]. While the underlying pathophysiological mechanisms are not fully clarified, a link between AS and ATTR has been proposed, as it has been reported that approximately 11% of patients with severe AS have underlying coexistent ATTR [1]. This may be due to a demographic drive, since both clinical entities are observed in the elderly, as well as a pathophysiological link, given the direct infiltration of the aortic valve by TTR, which may favour the development of AS.
Figure 1. The prevalence of cardiac amyloidosis in various populations. Reproduced with permission from John Wiley and Sons [1].
The bubble plot shows various population subgroups on the y‐axis and the prevalence of cardiac amyloidosis (CA) on the x‐axis, with each bubble size corresponding to the study sample size. The study of Zegri‐Reiriz et al in patients with carpal tunnel syndrome is represented with two bubbles (prevalence in the overall population and in patients with bilateral carpal tunnel syndrome plus left ventricular hypertrophy (LVH).
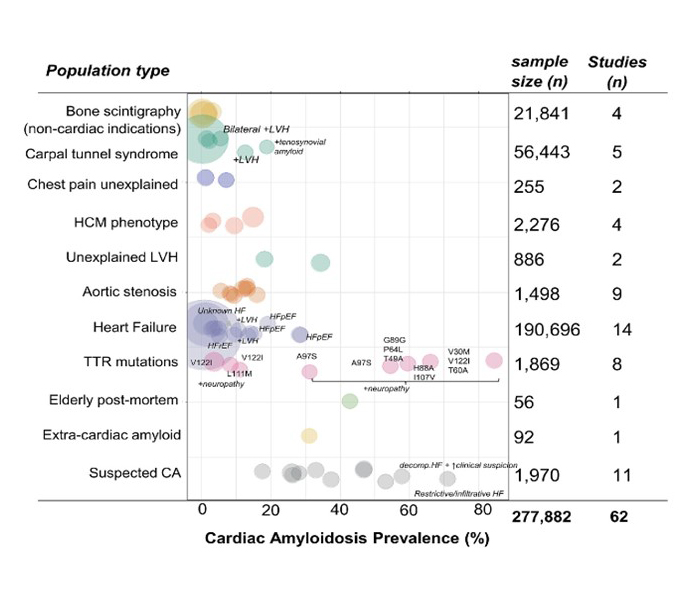
HCM: hypertrophic cardiomyopathy; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; TTR: transthyretin
In order to diagnose latent ATTR in specific groups of patients, increased vigilance for the disease is needed [6]. Diagnosing ATTR can be challenging, as ATTR can present with varying symptoms depending on the disease’s stage, subtype (wild-type vs hereditary), and with a wide variety of extracardiac manifestations [7-9]. Diagnosis of ATTR may be particularly challenging in specific patient subgroups like patients with severe AS, as the two clinical entities share common symptoms and a similar clinical phenotype, e.g., concentric left ventricular hypertrophy [4,10]. Consequently, heart failure symptoms, concentric hypertrophy and elevated natriuretic peptide levels in AS are typically attributed to valvular heart disease and ATTR remains underdiagnosed and undertreated, leading to increased rates of heart failure-related hospitalisations, morbidity and mortality [2]. A timely ATTR diagnosis can alter the natural course of disease since the initiation of TTR-specific treatment can improve prognosis and reduce hospitalisations [11].
Shifting concepts in ATTR: from a rare disease to a common cause of heart failure
ATTR has been traditionally viewed as a rare clinical entity. Clinical vigilance for disease diagnosis had remained low as no disease-specific treatment has been available. However, with the help of advanced imaging diagnostics and approval of TTR-targeted treatments, an increased interest in ATTR has emerged. Even though ATTR has increasingly been recognised, many patients still remain undiagnosed or are diagnosed late in the natural course of the disease, limiting the expected benefits of TTR-targeted treatments, such as TTR stabilisers [2, 12].
The population prevalence of ATTR cannot be accurately calculated; in fact, it largely depends on the patient subgroup being screened [1]. Patients with a phenotype of hypertrophic cardiomyopathy show a varying ATTR incidence from ~3% to ~15% [1]. Similarly, up to 15%-20% of patients with unexplained HFpEF and left ventricular hypertrophy may have concomitant ATTR [1]. Approximately 11% of patients with severe AS may also have underlying ATTR [1, 10, 13]. This suggests that screening for ATTR should be prioritised in specific patient groups, e.g., patients with severe AS, where ATTR prevalence is high. This could lead to the identification of large numbers of ATTR cases that may otherwise remain undiagnosed.
The challenges of diagnosing ATTR in the setting of severe aortic stenosis
Patients with a dual diagnosis of severe AS and concomitant CA are more frequently elderly males. The prevalence of ATTR increases with age. In patients >80 years old, ATTR prevalence is as high as ~18%, while in post-mortem studies of elderly individuals, cardiac deposition of TTR fibrils is typically found, even if no overt cardiomyopathy is present [14].
The significant overlap that is observed in both the symptoms and clinical presentation of the two diseases poses diagnostic challenges [4]. In the setting of AS, clinical suspicion for ATTR may be triggered by extracardiac findings such as spinal cord stenosis, bilateral carpal tunnel syndrome, ruptured biceps tendon, orthostatic hypotension, and lower extremities numbness [7-9]. Typical electrocardiography (ECG) findings in ATTR include conduction abnormalities (atrioventricular blocks, bundle branch blocks), prolonged QRS duration and atrial fibrillation, although these may be less specific in the setting of severe AS. A pseudoinfarction pattern and low QRS voltage-to-mass ratio are less commonly observed in AS and therefore their presence should raise suspicion for underlying ATTR [8 9,14].
Similarly, ECG findings such as left ventricular hypertrophy (LVH), mild pericardial effusion, thickened valves, low tissue Doppler imaging (TDI) velocities, apical sparing pattern and biatrial dilatation are reported as red flags for ATTR although these can be observed in lone AS too [7-9]. The presence of diastolic disfunction, elevated filling pressures and a presentation of HFpEF or HF with mildly reduced ejection fraction (HFmrEF) can be observed both in severe AS and in ATTR and does not flag CA in such patients [4]. Patients with severe AS typically have increased wall thickness and left ventricular hypertrophy (LVH) as a result of the increased left ventricular pressures that lead to concentric remodelling of the heart. ATTR patients, on the other hand, present with increased LVH caused by the deposition of amyloid fibrils in the myocardium. Thus, LVH lacks specificity for detecting ATTR in AS patients, as it can be present in both diseases [4].
However, among the findings that can raise suspicions of underlying ATTR are higher E/A and E/e΄ ratios. This echocardiographic marker is found significantly more frequently in patients with a dual pathology compared to patients diagnosed with lone AS [14]. Lower body mass index, low-flow low-gradient AS type and reduced ejection fraction are reported to be more frequently observed in patients with a dual pathology. Biomarkers can also raise suspicions of ATTR among AS patients. Elevations in NT-proBNP that are out of proportion to HF symptoms as well as persistent mild increases in hs-TnI levels may indicate underlying ATTR [14].
Advanced imaging by cardiac magnetic resonance (CMR) imaging is particularly helpful to discriminate ATTR cardiomyopathy from heart failure due to severe AS. Tissue characteristics including highly increased extracellular volume (even in the early disease stages of ATTR), increased native T1 mapping values and abnormal gadolinium kinetics of presenting with patterns like global subendocardial or transmural Late Gadolinium Enhancement (LGE) are not observed in AS and can confirm the diagnosis of cardiac amyloidosis.
Importantly, neither echocardiography nor CMR can be used to discriminate between ATTR and other subtypes of amyloidosis, like AL amyloidosis, and therefore, ATTR diagnosis must be confirmed by bone scintigraphy and haematological tests [7,15].
Beyond Ockham’s Razor: The clinical implications of a dual diagnosis of severe AS and ATTR
Although ATTR and AS are two distinct entities, common pathophysiological links may exist and further research in this direction is needed. The diagnosis of a concomitant ATTR in AS patients has clinical implications. Both clinical entities are associated with poor prognosis [2,10,13]. ATTR and severe AS also share common patient demographics, affecting mainly elderly patients (>65 years old) [10,13]. However, it has been shown that patients with a dual diagnosis have an even worse prognosis in terms of all-cause mortality [10]. This underlines the importance of diagnosing ATTR in this group of patients [10].
Despite the increased disease-awareness and the higher number of patients that are referred to expert amyloidosis centres in recent years for ATTR screening, ATTR remains significantly underdiagnosed among AS patients (Figure 2) [2]. We have previously shown that among patients undergoing transcatheter aortic valve implantation (TAVI) in the US, only ~1% have been diagnosis with ATTR, when the known incidence of ATTR in this group of patients is close to 11% [2]. This highlights the need to systematically screen TAVI patients for ATTR. Persistent HF symptoms and a poor clinical course after successful TAVI may indicate other reasons beyond valvular heart disease, such as coexistent ATTR [2,10,13]. Patients with a dual pathology, i.e., ATTR and AS, also tend to have a longer length of hospitalisation and higher hospitalisation costs during TAVI procedures, which underlines the clinical importance of diagnosing latent ATTR to improve patient prognosis and to reduce the relevant healthcare burden [2]. Other considerations include technical aspects of the procedure such as valve type, deployment height, and vascular access, to minimise complication risks in ATTR patients undergoing TAVI. Currently, evidence in this field is lacking and further research is required.
Figure 2. Prevalence of cardiac amyloidosis in patients with aortic stenosis undergoing transcatheter aortic valve implantation. Reproduced with permission from [1] and [16].
(A) Number of total hospitalisations each year from 2015 through 2019. (B) Total number of included patients undergoing transcatheter aortic valve replacement (TAVR) and observed/expected cardiac amyloidosis (CA) cases based on available published pooled estimates [1]. (C) Clinical profile of TAVR patients with CA and without CA. (D) In-hospital mortality rates for TAVR patients with and without CA. (E) Multivariable analysis for in-hospital mortality.
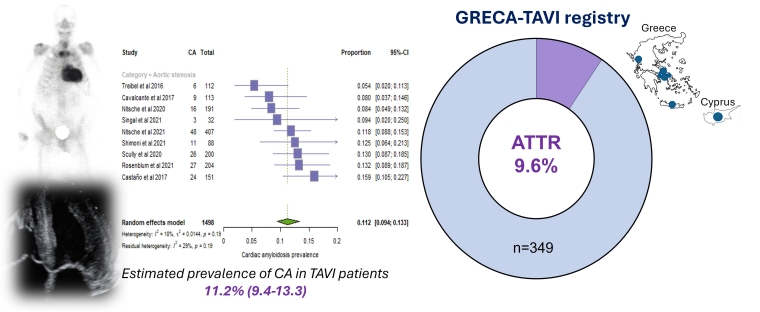
CI: confidence interval; Hospit: hospitalisation; LOS: length of stay; OR: odds ratio; PPM: permanent pacemaker
Nitsche et al [10] have proposed the use of the RAISE score (Remodelling, Age, Injury, Systemic and Electrical disorders) to detect ATTR in patients with severe AS [10,17]. This scoring system uses various predictors to calculate the probability of coexistent AS and ATTR and has demonstrated good performance in flagging these patients [10]. Scores ≥2 and ≥3 points have a sensitivity of 93.6% and 72.3%, and a specificity of 52.1% and 83.6%, respectively, for ATTR [10,17]. A RAISE score ≥2 points has been proposed as the optimal cut-off to initiate screening for ATTR by bone scintigraphy and a haematologic work-up [18, 19]. Possible alterations of the aforementioned score could further enhance its diagnostic ability to detect patients with a dual pathology. Adding ATTR red flags to the score, including biventricular hypertrophy, or tricuspid annular plane systolic excursion (TAPSE) <14 mm, has been proposed as a way to potentially modify the score [18].
Regardless of the coexistence of ATTR in severe AS [10,14,18-21], patients benefit from TAVI and treatment of valvular heart disease. Choosing TAVI compared to surgical aortic valve replacement (SAVR) seems reasonable given the high-risk profile of ATTR patients and to minimise the risk of periprocedural complications associated with surgical correction [19]. The efficacy of TTR-targeted treatment in patients post-TAVI has not been demonstrated, although currently there is no indication to withhold TTR-targeted treatments such as tafamidis in these patients, given their beneficial effects on both quality of life and hard clinical endpoints [2,13].
Conclusions - Impact on clinical practice
ATTR cardiomyopathy should not be viewed as a rare disease. ATTR can be the cause of heart failure in up to 15% of patients with HFpEF and hypertrophy, while approximately 11% of patients with severe aortic stenosis may have concomitant ATTR. Suspicion of ATTR in patients with aortic stenosis is challenging as both diseases share common symptoms and a similar imaging phenotype. Most typical clinical signs and findings for ATTR may lack specificity in coexistent severe aortic stenosis. Therefore, systematic screening for ATTR of patients with aortic stenosis over 65 years old in the presence of left ventricular hypertrophy (or other red flags) is currently recommended. Timely treatment of ATTR can attenuate progression to end-stage heart failure and improve quality of life and survival.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.