Keywords
Cardiovascular outcome trials
Cardiovascular risk
Diabetes management
Diabetes therapies
Renal risk
Abbreviation list
CVOT: cardiovascular outcome trial
FDA: U.S. Food and Drug Administration
GLP-1 RA: glucagon-like peptide-1 receptor agonists
SGLT2i: sodium-glucose cotransporter-2 inhibitors
Take-home messages
- Although glycaemic control is an essential factor in preventing and managing microvascular complications in diabetes mellitus, a more comprehensive approach is necessary in order to reduce the risk cardiovascular events.
- Since the FDA’s recommendations in 2008, the introduction of CVOTs has enabled the assessment of cardiovascular safety and benefits of new glucose-lowering agents.
- Initially approved as antidiabetic agents, GLP-1 RA and SGLT2i have demonstrated results past their glucose-lowering effect, providing additional cardiovascular and renal protection.
Introduction
Cardiovascular disease (CVD) and diabetes mellitus (DM) are two strongly interconnected conditions. Although their interaction is already well-known and their multiple links are extensively studied, over the course of the past few decades the primary focus in DM management has changed significantly. The early 20th century represented a turning point in recognising the implications of type 2 DM (T2DM), beginning with Hitzenberger’s and Richter-Quittner’s clinical observations, laying the groundwork for the understanding of what is now known as metabolic syndrome and its association with an increased cardiovascular (CV) risk.
Lessons from the past
Large-scale randomised controlled trials that evaluated the impact of glycaemic control on diabetes-related complications have a major common finding: while a strict glycaemic control can reduce microvascular complications and has a potential long-term legacy-effect, it does not appear to significantly impact macrovascular complications rates. In fact, some of them highlighted the increased risk of complications associated with a strict glycaemic control. The UKPDS’ (United Kingdom Prospective Diabetes Study) primary endpoint was to compare conventional versus intensive blood glucose control regarding any diabetes-related endpoints in a cohort of patients with T2DM. Its results have demonstrated a reduction in microvascular complications, but without any effect on mortality, and although a trend towards a decrease in macrovascular complications was observed, it lacked statistical significance [1].
The DCCT Diabetes Control and Complications Trial) evaluated the impact of achieving near-normal blood glucose levels on the frequency and progression of microvascular complications in patients with type 1 DM (T1DM). After a mean follow-up period of 6.5 years, patients in the intensive therapy group had a delay in the onset and progression of diabetic microvascular complications, emphasising the significance of tight glycaemic control in T1DM [2].
Following the UKPDS, in which the associations between HbA1c levels and the incidence of diabetes-related complications were observed, the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial aimed to evaluate the hypothesis that tighter glycaemic control (a target haemoglobin A1c level ≤6.5%) could result in lower risk of both micro- and macrovascular complications, with a primary endpoint of a composite of vascular outcomes. Although a reduction in major vascular events was seen, this result was mainly driven by a reduction in microvascular event rates, with no significant variations with respect to the occurrence of macrovascular events [3].
With a more ambitious glycaemic target, the ACCORD (Action to Control Cardiovascular Risk in Diabetes Study Group) trial had as purpose to establish if obtaining a normal haemoglobin A1c level (HbA1c <6.0%) would prevent the incidence of major CV events in adult patients with T2DM. Not only is it that intensive glucose-lowering therapy failed to reduce major CV and microvascular events, but it also increased mortality in this group [4].
The Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes trial (VADT) enrolled military veterans with poorly controlled T2DM and randomised them to receive either standard or intensive glycaemic control, with the primary outcome being the first occurrence of major CV events. The trial yielded similar results in terms of events, with no differences observed between the two groups in terms of major CV outcomes, mortality or microvascular complications. Nevertheless, the intensive control group demonstrated a reduction in the progression of albuminuria [5].
Taking into account the results from these landmark trials, it can be concluded that, although glycaemic control is an essential factor in preventing and managing microvascular complications in DM, a more comprehensive approach is necessary in order to reduce the risk of CV events.
Glycaemic control: weighing the costs
Rosiglitazone, an antidiabetic drug from the thiazolidinediones class, improves the target cells’ response to insulin, while preserving pancreatic beta cells. With glucose-lowering effects similar to metformin and sulfonylureas, and high effectiveness in terms of metabolic control by targeting the core pathogenic mechanism of insulin resistance, the drug was widely used in the treatment of T2DM. In 2007, Steven E. Nissen published a meta-analysis that included 42 trials conducted with rosiglitazone and evaluated the drug in terms of CV safety. Results showed a 43% increase in myocardial infarction rates in the rosiglitazone group [6]. Its popularity had a sudden drop with the publication of Nissen’s meta-analysis and serious concerns regarding the drug’s safety were raised, leading to a black box warning issued by the U.S. Food and Drug Administration (FDA) in November 2007.
One year later, the FDA imposed strict rules for ensuring CV safety of novel T2DM medication by requiring the conduction of long-term post-marketing outcome trials [7]. Until then, studies with glucose-lowering drugs were conducted with a shorter duration, typically 6 months to 1 year, and included mainly low-risk patients without CVD or additional CV risk factors. Their approval was primarily based on a reduction in HbA1c and did not include predefined criteria to analyse CV outcomes. In contrast, the FDA requirements regarding the conduction of long-term CV outcome trials (CVOTs) were specifically tailored to rule out unacceptable CV risk, with an upper bound of the 95% confidence interval (CI) for the risk ratio (RR) of major CV events of below 1.8 for pre-approval and below 1.3 for post-approval application. If the upper limit of the two-sided 95% CI for RR falls between 1.3 and 1.8 and the overall risk-benefit assessment supports approval, a post-marketing trial is necessary to demonstrate that the 1.3 non-inferiority margin is reached.
These requirements translate into longer trial durations and enrolment of a larger cohort in order to meet the approvability bar. In terms of the primary endpoint, CVOTs focus on a composite of major adverse CV effects (MACE), which include CV mortality, myocardial infarction (MI), and stroke. The duration of these trials is event-driven to provide sufficient data on long-term CV outcomes of new glucose-lowering medication, leading thus to observations extending for many years (usually more than 2 or 3 years, with trials being conducted even for 5 years or more). Additionally, CV events must be adjudicated independently and in a blinded manner in all Phase 2 and 3 trials, providing a higher confidence level. As for patient selection, to ensure safety-of-use in higher-risk populations, inclusion of elderly patients, with a longer disease duration, some degree of renal impairment, established CV disease or CV risk factors became mandatory [8].
The paradigm shift
Since the FDA’s recommendations in 2008, the introduction of CVOTs has enabled the assessment of new aspects related to CV risk in glucose-lowering medication. Over the years, several CVOTs were conducted with DPP-4 inhibitors, insulin, sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1-RA). Although all these classes indicated non-inferiority concerning MACE, SGLT2i and GLP-1 RA provided unexpected beneficial results regarding CV safety.
EMPA-REG OUTCOME was the first CVOT conducted with an SGLT2i, demonstrating that, in patients with T2DM and established CVD, empagliflozin significantly reduced all-cause mortality (P=0.002) and death from CV causes (P<0.001), showing superiority versus placebo in reducing the composite 3-point MACE (P=0.04). Additionally, significantly lower rates of hospitalisations for heart failure (HHF) were also observed (P=0.002) [9]. Canagliflozin’s CV safety was evaluated in the CANVAS trial, with a usual primary endpoint consisting of CV death, non-fatal MI, and non-fatal stroke. Results proved a reduction in MACE (P=0.02 for superiority), lower rates of HHF and additional renal benefits in the canagliflozin group [10]. Dapagliflozin proved non-inferiority versus placebo in reducing MACE (P<0.001 for non-inferiority) and superiority in reducing a composite of CV death and HHF (P=0.005) in the DECLARE-TIMI-58 trial which included patients with T2DM that had or were at risk for atherosclerotic CVD (ASCVD), but with a trial population that could explain the difference in results when compared to the previously-mentioned trials [11].
Regarding the GLP1-RA, three agents provided promising results in CVOTs conducted with this glucose-lowering medication. Liraglutide was the first GLP-1 analogue evaluated for CV safety in the LEADER trial and proved superiority in reducing the composite 3-point MACE (P=0.01) and in reducing the rate of microvascular events (P=0.02), mainly nephropathy (P=0.003) [12]. The SUSTAIN-6 trial investigated once-weekly injectable semaglutide concerning CV safety and its results indicated a significant reduction in MACE in the semaglutide group (P<0.001 for noninferiority), outcome driven mostly by a reduction in stroke rates (1.6% versus 2.7%, P=0.004) [13].
Expanding horizons of diabetes medications
The results of CVOTs conducted with SGLT2i and GLP1-RA surpassed expectations, indicating their potential benefits beyond mere metabolic control, prompting further investigation into the underlying mechanisms. Initially approved as antidiabetic therapies, these molecules exhibited results past their glucose-lowering effect, providing additional CV and renal protection. A consistent finding in CVOTs conducted with SGLT2i was a reduction in HHF rates, indicating that these agents have a cardioprotective effect and target several key pathways implicated in heart failure (HF).
DAPA-HF was the first trial to evaluate the effect of an SGLT2i in patients with chronic HF with reduced ejection fraction (HFrEF), regardless of the presence of T2DM. The primary outcome was defined as a composite of CV death and worsening of HF, which included hospitalization or an urgent visit requiring intravenous therapy. Dapagliflozin reduced the risk of CV death and worsening of HF in patients with HFrEF, both in patients with and without diabetes (P<0.001) [14]. EMPEROR-Reduced demonstrated similar results with empagliflozin in terms of primary outcome (P<0.001), along with a slower decline in the estimated glomerular filtration rate (eGFR) [15]. The positive results yielded in HFrEF prompted investigators to also conduct studies in patients with HF with mildly reduced (HFmrEF) and preserved ejection fraction (HFpEF). These conditions are characterised by an impaired ventricular filling and diastolic disfunction, a common finding among elderly individuals with hypertension, T2DM and CKD. In the DELIVER trial, dapagliflozin demonstrated a reduction in the combined risk of CV death and worsening of heart failure in patients with chronic HF with mildly reduced and preserved EF (P<0.001) [16]. Empagliflozin yielded the same results in the EMPEROR-Preserved trial, demonstrating beneficial effects in patients with or without T2DM and an EF above 40% (P<0.001) [17]. The results of these trials indicate that the benefits of SGLT2i in HF are evident irrespective of the EF value and regardless of the presence of T2DM, highlighting that the cardioprotective effects of these agents are not solely attributed to glycaemic control, instead they reinforce the involvement of other complex mechanisms.
In addition to their benefits in HF, SGLT2i have also shown renal benefits, a hypothesis further tested in several trials conducted on patients with CKD. DAPA-CKD demonstrated that patients treated with dapagliflozin had a significantly reduced risk of a composite outcome comprising of an eGFR decline of at least 50%, progression to end-stage kidney disease, or death from renal or CV causes (P<0.001). Moreover, these benefits were consistent across patients with and without T2DM [18]. With a similar study population, EMPA-Kidney compared empagliflozin to placebo in reducing the risk of progression of kidney disease or death from CV causes. The results indicated that empagliflozin significantly lowered the risk of kidney disease progression along with lower rates of CV death, demonstrating a substantial reduction in the primary composite outcome (P<0.001) [19]. Consequently, these findings underscore the potential of SGLT2i as a treatment option for patients with HF and/or CKD, independent of their diabetes status, EF, or degree of renal impairment, thereby expanding their use in clinical practice (Figure 1).
Figure 1. Evolution of the treatment recommendations in type 2 diabetes management.
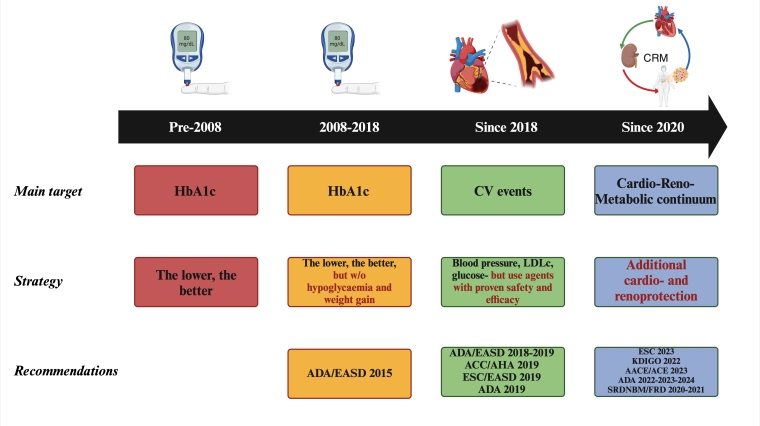
AACE: American Association Of Clinical Endocrinologists; ACC: American College of Cardiology; ACE: American College Of Endocrinology; ADA: American Diabetes Association; AHA: American Heart Association; EASD: European Association for the Study of Diabetes; ESC: European Society of Cardiology; FRD: Federația Română de Diabet; HbA1c: glycated haemoglobin; KDIGO: Kidney Disease Improving Global Outcomes; LDLc: low-density lipoprotein cholesterol; SRDNBM: Societatea Română de Diabet, Nutriție și Boli Metabolice
The conclusion derived from CVOTs conducted with GLP1-RA is that these molecules demonstrate an anti-atherosclerotic effect independent of weight reduction, through various mechanisms that have not yet been fully elucidated. Furthermore, a consistent finding in trials involving these agents was significant weight loss, attributed to delayed gastric emptying, and modulation of hormones and neurotransmitters involved in appetite regulation. This effect has led to the approval of certain molecules of the GLP1-RA class, such as semaglutide and liraglutide, for the treatment of obesity, regardless of the patient’s glycaemic status.
When comparing the two classes of antidiabetic drugs, while the effects of the SGLT2i mostly involve changes in energy metabolism and haemodynamics, the protective effects of GLP1-RA are mainly anti-atherosclerotic. Although these classes have different mechanisms of action, they exert complementary effects, with both cardio- and renoprotective benefits, extending their use in patients with CVD and/or CKD, regardless of the presence of T2DM.
Taking into consideration these results, current ESC Guidelines on Diabetes and CVD, published in 2023, brought major revisions regarding risk stratification and treatment recommendations in patients with T2DM. In individuals without CVD or severe target organ damage (TOD), CV risk was assessed using data on disease duration, renal function, and the presence of additional CV risk factors. However, new guidelines introduced a novel algorithm specifically tailored for patients with T2DM, SCORE2-Diabetes, which considers geographic variation alongside diabetes-specific variables and traditional risk factors, aiming to provide a more accurate CV risk stratification for individuals with T2DM without established CVD or TOD. Additionally, to reduce CV risk, the use of SGLT2i and/or GLP1-RA, independently of HbA1c levels and concomitant glucose-lowering medication, is recommended. Given the mechanisms that provide CV protection, GLP1-RA are suited for patients with T2DM and atherosclerotic CVD (ASCVD), while SGLT2i, by reducing HF and renal endpoints, are currently recommended in patients with HF and/or CKD, although they have also proven beneficial effects in patients with ASCVD or an increased risk of developing ASCVD [20].
Conclusion/Impact on daily practice statement
In a population already at increased risk of developing CVD, the management of patients with T2DM requires a comprehensive approach and targeted interventions. While glycaemic control remains a cornerstone of T2DM treatment, a tailored approach towards additional CV and renal protection is needed, leading to current recommendations which emphasise the need of employing - in all patients - diabetes therapies with additional cardiovascular and renal benefits, irrespective of the quality of glycaemic control or the achievement of the glycaemic targets.




 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.