Introduction
There are two basic scenarios in telemedicine in heart failure (HF) patients (synonym: telecardiology): (1) to enable an exchange of diagnostic information between different medical providers themselves (so-called "doc-to-doc telecardiology"), and (2) for a direct connection between a doctor and a patient in his/her home (so-called ”doc-to-patient telecardiology”). Both scenarios can help the primary treating physician to follow up patients with HF.
“Doc-to-Doc” telecardiology
The telemedical information and communication technology (ICT) enabling exchange between physicians is mostly based on a telemedical transfer of image data, e.g., echocardiographic findings of rare diseases (for example, in adults with congenital heart defects or in cardiomyopathies in the context of cardiac involvement in systemic diseases, such as Fabry's disease). This type of telemedical use is usually combined with a videoconference and time-shifted to the clinical examination of the patient. Due to increasingly cost-effective, small and transportable systems (e.g., an ultrasound transducer as a supplement to a tablet), such diagnostics can also be performed by family doctors or HF nurses as point-of-care diagnostics for focused questions, e.g., as part of home visits. Therefore, this method is particularly suitable for screening and follow-up for HF patients in rural areas with sparse access to medical care providers.
“Doc-to-Patient” telecardiology
The second scenario is a connection between cardiologists and patients themselves in their homes using telemedical measurement devices and other ICT infrastructure. This care concept is an addition to the existing outpatient care provided by a general practitioner (GP) and cardiologist.
The principles of the doctor-patient relationship differ in one feature between telemedicine and traditional HF outpatient care, i.e., the lack of direct physical contact. All other characteristics, such as the provision of personal medical care, medical standards, medical due diligence and confidentiality are basics in telemedical HF care. However, these criteria define the difference between so-called “HF service centres” and a “telemedical centre (TMC)”.
Telecardiology in the care of HF patients
Telemedicine in HF is rapidly evolving [1] and both described scenarios are in use. For example, in HF patients on vacation (e.g., on cruise ships), findings between medical service providers on board and the TMC can be exchanged (doc-to-doc).
However, direct communication between patient and TMC is more common (doc-to-patient). The patient measures daily vital signs at home according to a defined measuring plan, such as electrocardiogram (ECG), blood pressure and weight, and transmits them to the TMC. In this way, cardiac decompensation can be detected early, and further deterioration may be prevented, e.g., by adjusting the medication.
Technical implementation
There is a huge variety of telemonitoring devices, which can be roughly divided into implantable/ invasive devices and non-invasive devices.
Depending on the underlying device technologies, the onset of cardiac deterioration can be detected at different time points in the course of disease ideally to prevent a clinical manifestation of HF decompensation and HF hospitalisation [2].
Telemonitoring with cardiovascular implantable electronic devices (CIED) provides diagnostic information about early markers regarding the onset of pulmonary congestion, e.g., an elevated left ventricular end-diastolic pressure (LVEDP) or elevated left atrial pressure (LAP), a decreased intrathoracic impedance, changes in pulmonary artery pressure (PAP), and arrhythmias.
Non-invasive devices such as scales and blood pressure monitors have a “late” sensitivity compared to implanted telemonitoring devices. To compensate for this systematic deficit of non-invasive devices, the review of transferred vital parameters should be performed the same day, which requires a 24/7 telemedical service. However, there are also advantages of non-invasive devices, e.g., low costs and the absence of inherent safety concerns of implantable devices.
In Germany, a randomised clinical trial (RCT) evaluating the benefits of PAP measurement versus non-invasive telemedical intervention is currently underway (acronym: PASSPORT-HF, NCT04398654).
Nevertheless, all types of telemonitoring systems require the possibility to initiate an appropriate medical treatment immediately.
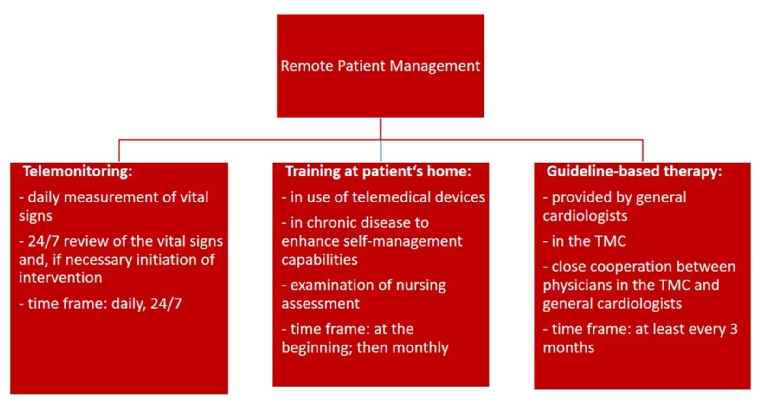
The term “remote patient management (RPM)” describes a holistic digital concept in the outpatient care of HF patients. This concept contains three parts (Figure 1) [3]:
- Guideline-based therapy provided by GP, cardiologists and HF nurses.
- Nurse-based training at patient’s home: training regarding the use of medical devices (including installation), disease-related self-management and nursing assessment (e.g., description of self-management capabilities, social integration). This training will be followed by a standardised telephone call every month.
- Telemonitoring: daily and independently, the patient measures vital parameters at home and transmits them to the TMC. The data will be reviewed 24/7 in the TMC. In case of crossing thresholds of patient-specific cut‐off limits and in case of emergency, the medical staff intervene, e.g., by changing medication, recommendation of outpatient consultation or alerting an ambulance.
Figure 1. RPM concept for HF patients.
The technical implementation of telemedical HF care consists of the following elements:
- Invasive measuring devices, e.g., implants for measurement of atrial pressure or detection of events such as syncope.
- Non-invasive measuring devices, e.g., scales or blood pressure monitors with interface for wireless data transfer (e.g., Bluetooth Low Energy [BLE], near field communication [NFC]).
- Data protection compliant, encrypted data transfer between patients and the telemedical staff.
- IT infrastructure at the telemedical service provider (e.g., electronic health record [EHR]).
The flow and processing of medical data exchanged between HF patients and telemedical staff represents another classification system.
The telemedical information flow can be divided into an afferent and an efferent arm.
In the afferent arm, the patient transmits data to medical staff, while in the efferent arm the medical staff send the results of the review back to the patient.
Irrespective of the specific technical system architecture, the afferent information flow is mostly identical in all telemedical settings due to the measurement and transfer of vital parameters. The fundamental difference concerns the processing of diagnostic information in the efferent way. There are currently three different telemedical settings implemented for HF patients.
Telemonitoring via web-based database accessed by attending physician
The vital signs are transferred from the patient’s home to a web-based EHR. The attending physician can access and review these data and, if necessary, recommend inpatient or outpatient visits for further examination or intervention. This is very common for the follow-up of implantable devices. The efficiency and security of this telemonitoring concept have been proven in various RCTs. Nevertheless, the web portals are manufacturer-dependent proprietary systems, which require individual training for medical staff. The biggest disadvantages are the lack of interoperability of these systems and the fact that these systems are not designed and approved for emergency care.
Telemonitoring by a medical call centre
In recent years, medical call centres have been established to care for patients with chronic diseases, even for HF patients. Most of these centres are owned by health insurance companies or commercial companies and are using non-invasive devices. The vital parameters measured by the patients are transferred to an EHR at the call centre, including patient-specific cut-off limits. When crossing these thresholds, the medically trained staff at the call centre forward this information to the primary physician, who decides about further diagnostics and interventions. As a result, this form of telemonitoring can only be provided during office hours, which can cause a considerable time delay between the detection of a threshold crossing event and the initiation of intervention, in particular at weekends and bank holidays. According to their websites, some call centres offer 24-hour advice from healthcare professionals, but no emergency care. This type of telemedicine is very suitable for those patients with chronic diseases, where responses within one or two days are considered sufficient when significant changes in vital parameters are observed. One example is outpatient medication titration at the beginning of hypertension therapy. For telemedical care of HF patients, the medical call centre model is not optimal, since changes in their clinical status require a quick response. The main responsibility for the patient’s therapy remains with the GP and cardiologists; the call centres provide only an additional service to them.
RPM provided by a TMC
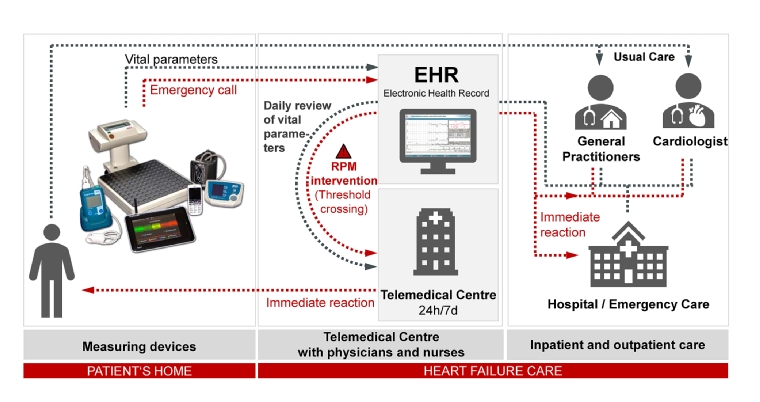
The TMC represents a department of a third level hospital (e.g., a university clinic) with departments to treat the most common comorbidities of HF (e.g., stroke, depression, kidney dysfunction, diabetes) and to carry out consultations. A TMC is staffed with physicians and HF nurses 24 hours a day, seven days a week (24/7) to receive and review vital parameters, obtained with non-invasive or invasive devices from different manufacturers. Moreover, the TMC should be part of a regional multidisciplinary HF network for close collaboration between cardiologists and other experts [4].
All the relevant patients’ reports, such as last hospital discharge reports or current laboratory reports, are stored in an EHR, and vital signs are reviewed with consideration of this information.
Initiated interventions range from medication adjustment in close cooperation with the GP and primary cardiologists to the recommendation of an unplanned outpatient visit or inpatient admission. In emergency situations and outside the practice opening hours, the medical staff of the TMC can act without prior contact with the primary treating physicians, who will be informed afterwards and receive regular historical reports (Figure 2).
Figure 2. TMC concept for HF patients.
EHR: electronic health record
This model of telemedicine is suitable for the outpatient care of high-risk HF patients with a required response time to changes in clinical status of less than one day. Application examples are the telemedical support of patients after discharge from an HF hospitalisation or patients with a permanent mechanical cardiac support system.
Clinical evidence
Within the last 15 years, many RCTs have been conducted to demonstrate the efficacy of RPM to reduce morbidity and mortality [5, 6]. Due to the diversity of the telemedicine systems as described above and different study designs (duration, patient profiles) the results are inconsistent. However, it can be concluded that RPM patients recently hospitalised for HF had fewer unplanned (cardiovascular) hospitalisation and days in hospital, a lower all-cause/cardiovascular mortality and improved quality of life compared to patients with usual care only [6]. An overview of the largest invasive and non-invasive randomised telemedicine trials finished since 2010 is given in Table 1.
Table 1. Overview of clinical trials in RPM for HF patients.
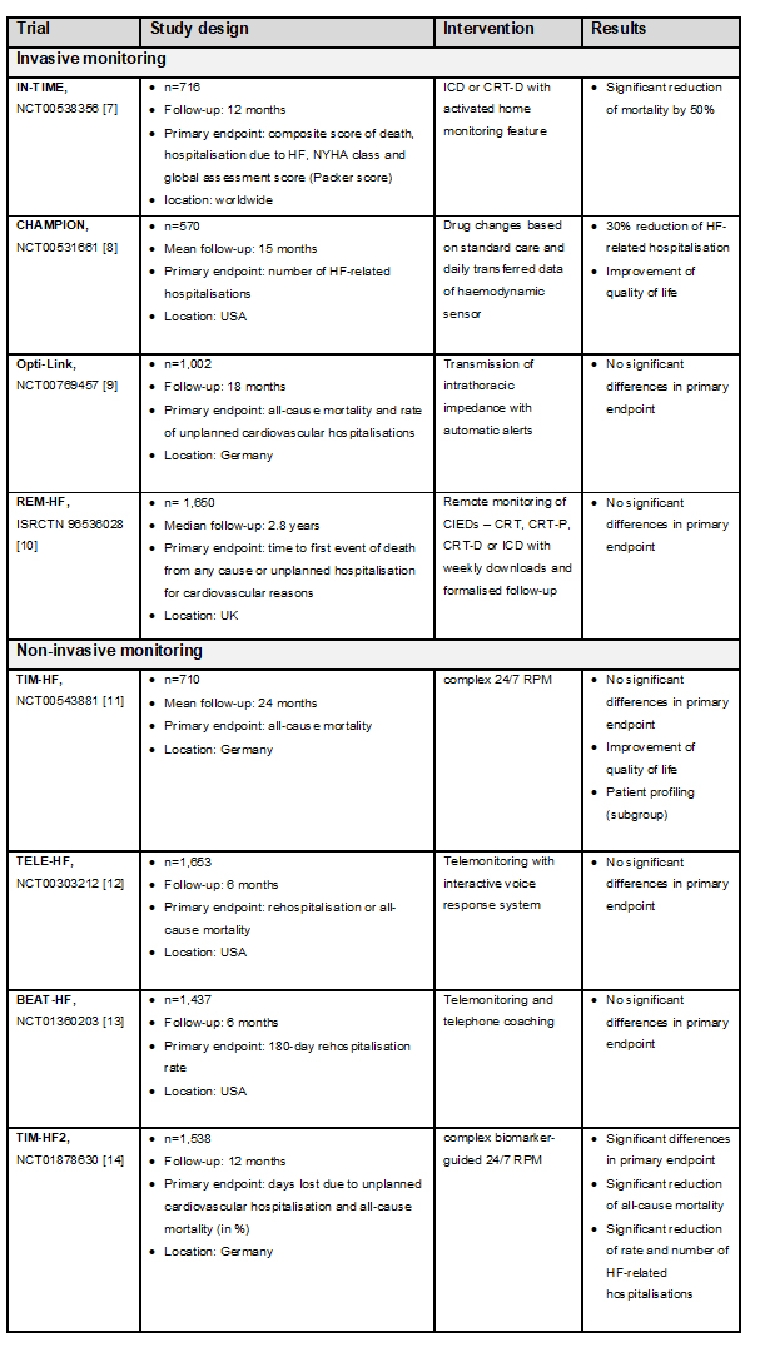
CIED: cardiac implantable electronic device; CRT: cardiac resynchronisation therapy; CRT-D: CRT device with defibrillator function; CRT-P: CRT with pacemaker; HF: heart failure; ICD: implantable cardioverter-defibrillator; RPM: remote patient management
The randomised, multicentre “Telemedical Interventional Management in Heart Failure II (TIM-HF2)” trial showed for the first time a significant improvement in all-cause mortality based on a non-invasive RPM [14]. The study results showed that telemedicine patients had to spend fewer days in hospital due to unplanned cardiovascular admissions - on average, 3.8 days p.a. compared to 5.6 days p.a. in the usual care group. As a result, these patients have lost significantly fewer days in relation to the one-year study period due to unplanned cardiovascular hospitalisations or all-cause death - 17.8 days compared to 24.2 days in the usual care group. In terms of all-cause mortality, out of 100 HF patients in the year of care, only eight patients of the RPM group (7.8 per 100 patient-years) died, while eleven patients died under the usual medical care conditions (11.3 per 100 patient-years).
Further post hoc analysis of TIM-HF2 identified positive effects on all-cause mortality in patients with atrial fibrillation [15] and recommended patient profiling via biomarker guidance. When excluding patients with all TIM-HF2 inclusion criteria and NT-proBNP <413.7 pg/ml and MR-proADM <0.75 nmol/L (representing 27% of the TIM-HF2 population), the number needed to treat with RPM to reduce all-cause mortality would decrease from 28 to 20 [16].
These results demonstrate that non-invasive RPM in selected cardiological patients leads to longer life and fewer and shorter hospital stays. These results were independent of the patients’ place of residence. Thus, the RPM concept is also suitable to compensate for regional differences in medical care for patients with chronic HF between urban and rural areas.
Guidelines of the European Society of Cardiology
In the current treatment guidelines of the European Society of Cardiology (ESC) for acute and chronic HF, a class IIb recommendation for invasive telemonitoring was established [17]. In 2019, a clinical update from the ESC’s Heart Failure Association (HFA) proposed considering an RPM concept such as in TIM-HF2 as part of the medical care of HF patients [18].
Current evidence about the clinical benefit of RPM is available for a duration of 12 months. The question as to the optimal duration of RPM remains unanswered [6]. An analysis in TIM-HF2 patients one year after the end of the RPM intervention showed no sustained effect of the intervention on mortality and morbidity under real-world conditions, although, at the end of the RPM intervention, telemedical patients showed a higher self-management capacity. This leads to the assumption that RPM has a positive effect only as long as it is performed [19].
Additionally, there is no evidence for RPM benefits relative to different healthcare systems.
Further developments
The next challenging step for the RPM concept is the translation from study conditions to usual care in a “real-world” setting. Currently, the TMC capacity is about 500 HF patients. For example, in Germany with approximately 200,000 patients meeting the TIM-HF2 inclusion criteria, more than 200 TMCs would be necessary. Technological innovations such as artificial intelligence (AI) are needed for upscaling this concept. Also, the use of a clinical decision support system in the TMC to support medical staff in their daily medical workflow is required.
Moreover, new sensor technologies are under investigation, e.g., AI-based voice analysis for the diagnosis of cardiac decompensation [20].
The Apple Heart Study (NCT03335800) was the first large-scale non-randomised, site-less, uncontrolled, pragmatic study with wearables and smartphones to show algorithm-identified possible atrial fibrillation [21]. The aim of the LINK-HF trial (NCT03037710) was the examination of a personalised analytical platform performance using continuous data streams to predict HF rehospitalisation. The algorithm detected precursors of HF hospitalisation with 76% to 88% sensitivity and 85% specificity [22].
However, there are currently no RCTs evaluating the reliability and validity of the data measured by wearables and smartphones. In addition, the increasing number of apps and devices makes it difficult to assess their risks and strengths. Data protection and data processing security issues have to be reassessed for each new application.
Conclusion
Telemedicine in HF patients is considered to be a relevant innovation that improves conventional HF outpatient care. The most beneficial HF patient group is patients at high risk, who were recently hospitalised due to HF in functional classes II and III, which represents approximately 20% of the total HF population. For the majority of stable HF patients or end-stage HF patients, there is no evidence of a beneficial effect of RPM. The introduction of telemedicine for a subset of HF patients will strengthen the cooperation among the GP, cardiologists and the third level HF units. Further research in the field is ongoing, in particular concerning the impact of AI technologies supporting telemedical HF care.




 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.